Chemistry Classification of Elements and Periodicity in Properties
Get insights from 75 questions on Chemistry Classification of Elements and Periodicity in Properties, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Classification of Elements and Periodicity in Properties
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
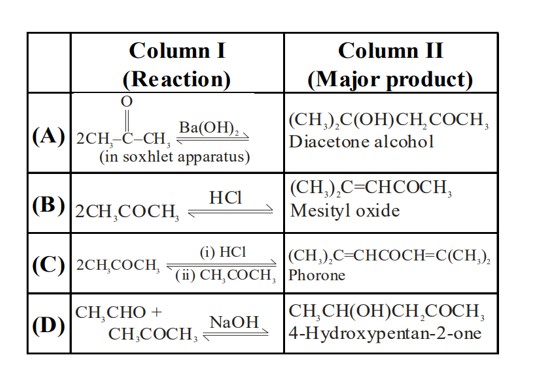
Yield of 4-hydroxypentan-2-one is low because aldol and diacetone alcohol are also formed.
New answer posted
3 months agoContributor-Level 10
Since sulphar has most -ve electron gain enthalpy hence S- will have the highest IE.
New answer posted
3 months agoContributor-Level 10
The second ionization energy of K is maximum, because the second electron is removed from fully filled 3p? subshell. The second ionization energies of group 2 elements decrease down the group.
Hence, second I.E. of Ca > second I.E. of Ba.
New answer posted
3 months agoContributor-Level 10
Al < Mg < Si < S < P
1st I. E increase along period with exception on moving from group 2 to group 13 and group 15 to group 16
New answer posted
4 months agoContributor-Level 10
Among isoelectronic monoatomic species, size is inversely proportional to atomic number. Hence among isoelectronic species Na? , O²? , N³? , F? (having the nearest noble gas configuration);
Order of size is Na? < F? < O? < N?
N³? has least atomic number hence the largest size
New answer posted
4 months agoContributor-Level 10
The difference between the first and second ionization energies is significantly higher for alkali metals (like Sodium, Na) compared to alkaline earth metals (like Magnesium, Mg). Therefore, in the context of the problem, X=Na and Y=Mg.
New answer posted
4 months agoContributor-Level 10
The atomic numbers and classifications for the given elements are: As (Arsenic, atomic no. 33) is a metalloid, I (Iodine, atomic no. 53) is a non-metal, and Bi (Bismuth, atomic no. 83) is a metal.
New answer posted
4 months agoContributor-Level 9
First ionization enthaly of Mg is smaller than Ar and Cl but higher than Na.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers