Chemistry Classification of Elements and Periodicity in Properties
Get insights from 75 questions on Chemistry Classification of Elements and Periodicity in Properties, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Classification of Elements and Periodicity in Properties
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Ionization enthalpy: B < Be < O < N
IE of N > O [Due to half-filled configuration]
IE of Be > B [Due to penetration effect]
New answer posted
5 months agoContributor-Level 10
Ionic radius of isoelectronic species depends upon ratio; Hence Ionic radius of ionic radius of Mg+
New answer posted
5 months agoContributor-Level 10
Ionisation enthalpy order :
Li > Na > K
He > Ne > Ar > Kr > Xe > Rn
Sr > Rb
Zn > Ga
New answer posted
5 months agoContributor-Level 10
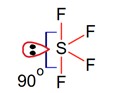
Geometry of SF4 is trigonal bipyramidal, in which there is one lonepair which occupy equatorial position as,
There are two lone pair – bond pair repulsions at 90°
New answer posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers