Chemistry NCERT Exemplar Solutions Class 11th Chapter Eight
Get insights from 125 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Eight, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Eight
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
So; x = 36
New answer posted
5 months agoContributor-Level 10
Initially -> 1 mol -
At eq. 1-x mol 2x mol
Here; molecules of Cl2 = atoms of Cl
i.e moles of Cl2 = moles of Cl
So : 1 – x = 2x x = 1/3
Moles of Cl2 at equibrium =
Moles of Cl at equilibrium =
Total moles =
No
New answer posted
5 months agoContributor-Level 10
A (g) -> B (g) KP = 100
at 300 K and 1 atm
Using
=-R * 300 * 2 * 2.3
So; x = 1380.
New answer posted
5 months agoContributor-Level 10
Stability constant are :
K1 = 104
K2 = 1.58 * 103
K3 = 5 * 102
K4 = 102
Overall stability constant K will be
K = K1 * K2 * K3 * K4
= 7.9 * 1011
Now, overall equilibrium constant for dissociation of [Cu (NH3)4]2+ is
= 1.26 * 10-12
So; x = 1 (Rounded off to the nearest integer)
New answer posted
5 months agoContributor-Level 10
Beo & Be (OH)2 are amphoteric in nature, because they react with both acid and base
New answer posted
5 months agoContributor-Level 10
Methane gas is produced during anaerobic degradation of vegetation that leads to global warming so considered as greenhouse gas like CO2. Also it causes cancer.
New answer posted
5 months agoContributor-Level 10
For stabilization of a - helix structure of protein, H-bonding is responsible which is in between group present in protein.
New answer posted
5 months agoContributor-Level 10
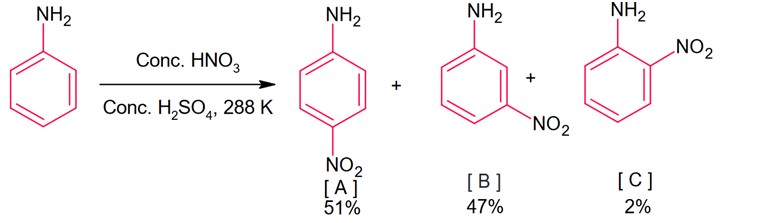
During nitration of aniline, meta product is also formed, it is because of due to presence of anilinium ion. In anilium group is meta directing group.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers