Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight
Get insights from 128 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
37. (D) Mn2+ acts as auto catalyst
Mn2+ formed in the reaction acts as an autocatalyst. Auto catalyst is a compound that is one of the products that are formed in the reaction itself catalyses the reaction.
Reaction: 2MnO4- + 16H+ + 5C2O42- → 2Mn2 + + 10CO2 + 8H2O
New answer posted
7 months agoContributor-Level 10
36. (A)i, ii
Disproportionation/redox reaction is a reaction in which one compound is oxidised as well as reduced at the same time.
(A) Cu+ ? Cu2+ + Cu
( + 1) ( + 2) (0)
(B) 3MnO4- + 4H+ ? 2MnO4- + MnO2 + 2H2O
( + 6) ( + 7) ( + 4)
New answer posted
7 months agoContributor-Level 10
35. (B)+3
Out of all +3 is the most common oxidation state for all the lanthanide elements.
New answer posted
7 months agoContributor-Level 10
34. (B) 3d5
Magnetic moment of an electron/dipole moment is caused by its intrinsic properties of spin and electric charge. It depends upon the number of unpaired electrons in its valence shell. The more the number of unpaired electrons, the greater will be the value of magnetic moment.
3d7 = 3 unpaired electrons
3d5 = 5 unpaired electrons
3d8 = 2 unpaired electrons
3d2 = 2 unpaired electrons
Out of all, 3d5 has five unpaired electrons that is maximum and hence it has the highest magnetic moment.
New answer posted
7 months agoContributor-Level 10
33. (A) Mn2O7
2KMnO4 + 2H2SO4 (conc.) → Mn2O7 + 2KHSO4 + H2O
Manganese heptoxide is an acid anhydride of permanganic acid ( HMnO4). It is a dangerous oxidiser, volatile and highly reactive liquid.
New answer posted
7 months agoContributor-Level 10
32. (B) CuF2
(A) Ag2SO4 (Ag+ )→ 5d106s0
(B) CuF2 (Cu2+ )→ 3d94s0
(C) ZnF2 (Zn2+)→ 3d104s0
(D) Cu2Cl2 (Cu+)→ 3d106s0
Unpaired electrons present in any compound impart colour to the salt of transition metal. Only CuF2 has unpaired electrons in its 3d orbital that's why it is white coloured in its solid-state while rest of the salts are colourless.
New answer posted
7 months agoContributor-Level 10
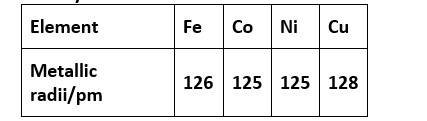
31. (D) Cu
Density is mass by volume. As we move from left to right for a long period, the atomic radii decrease. Hence, volume decreases. Also, an increase in atomic masses is observed.
So overall the density increases out of the above option from iron to copper, copper will have the highest density.
New answer posted
7 months agoContributor-Level 10
30. (A) Cu (II) is more stable .
Electronic configuration of Cuis [Ar] 3d10 4s1
Cu (I) - [Ar] 3d10 4s0
Cu (II)- [Ar] 3d9 4s0
Despite the fact that Cu (I) has fully filled 3d-orbital but Cu (II) is more stable than Cu (I) due to the greater effective nuclear charge of Cu (II) as nucleus has to hold 17 electrons rather than 18 electrons like in Cu (I).
New answer posted
7 months agoContributor-Level 10
29. (B) 26
Positive oxidation states indicate the loss of electrons from the atom. If X is in +3 oxidation state, then three electrons have been removed from it. Find the atomic number of the parent atom, we will add three in the given electronic number. i.e.
X3+ (Z = 23) = [Ar] 3d5
X = 23 + 3 = 26
New answer posted
7 months agoContributor-Level 10
28. Ionisation enthalpies are the main factor that influence the reactivity of transition elements. Higher the ionisation enthalpy, lesser is the reacting of the transition element.
When we move along the period from Sc to Cu, a regular increase in the ionisation enthalpy is observed which results in the almost regular decrease in the reactivity of elements.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers