Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight
Get insights from 128 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Eight
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Moles of chlorine in the given compound = Moles of chlorine in AgCl
= moles of AgCl
Mass of chlorine =
= 0.098 g
New answer posted
6 months agoContributor-Level 10
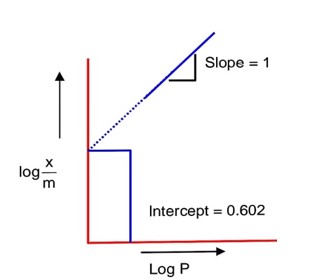
Using, Freundlich adsorption isotherm ;
………………. (i)
Comparing with y = mx + C
Slope =
Intercept, log k = 0.602
log k = log 4
k = 4
from equation (i)
= 0.12
= 12 * 10-2
So, 12 * 10-2 g of gas is adsorbed per gram of adsorbent,
New answer posted
6 months agoContributor-Level 10
Initial temperature ; T1 = 300 K
Final temperature ; T2 = 309 K
Rate constant gets doubled i.e K2 = 2K1
New answer posted
6 months agoContributor-Level 10
For the given cell, net redox reaction is as;
n = 2
Using;
Now, for the reaction
n = 1
So;
=
New answer posted
6 months agoContributor-Level 10
0.001 M NaOH solution has [OH-] = 0.001 M = 10-3 M
Using ; pOH =
=
pOH = 3
pH = 14 – pOH
pH = 14 – 3
pH = 11
New answer posted
6 months agoContributor-Level 10
Mass of solute ; wB = 2.5 * 10-3 kg
Mass of solvent,
Boiling point of solution ;
Boiling point of water ;
So, elevation in boiling point,
Using ;
Molar mass of solute ; MB = 45 g/mol
New answer posted
6 months agoContributor-Level 10
Number of moles, n = 5 mol
Temperature, T= 300 K
Initial volume, V1 = 10L
Final volume, V2 = 20 L
Using;
Work done; w = -2.303 nRT log10
= -8630 J
So, magnitude of work done is 8630 J.
New answer posted
6 months agoContributor-Level 10
n = 1, 2, 3 …….;
A. n = 3
is incorrect as
B. n = 3 = -2 is correct set.
C. n = 2
= +2 is incorrect set as
So; correct set of quantum numbers is B and C.
New answer posted
6 months agoContributor-Level 10
Determining empirical formula of the given compound.
Mass Moles Simplest ratio
C 41.8g &n
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers