Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven
Get insights from 89 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Increasing order of acidity is: ethanol < water < phenol.
This is because the phenoxide ion obtained after the removal of H+ is resonance stabilised, while the ethoxide ion obtained after the removal of H+ is destabilised by +1 effect of ethyl group. Thus phenol is a stronger acid than ethanol.
Now, ethanol is a weaker acid than water because the electron releasing ethyl group increases the ethanol density on oxygen and consequently the proton will not be released easily. There is no such effect is water.
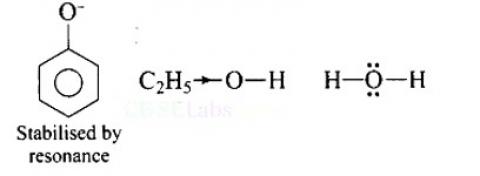
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
This can be explained as under:
(i) In phenol, the conjugation of unshared electron pairs over oxygen with aromatic ring results in partial double bond character in C – O bond.
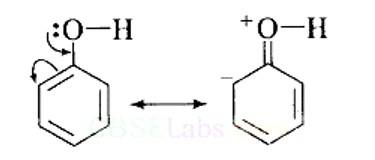
In methanol, no such conjugation (resonance) is possible.
(ii) In phenol, oxygen is attached to sp2 hybridised carbon while in methanol, oxygen attached to sp2 hybridised carbon. An sp2 hybridised carbon is more electronegative (because of greater 5-character) than sp3 hybridised carbon atom. Therefore, the bond between oxygen and sp2 hybridised carbon is more stable than the bond between oxyge
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Due to the presence of intermolecular H- bonds in alcohol the energy required to break H – bonds is more than the energy required to break simple dipole bonds present in ethers . Thus, the boiling point of alcohol is higher than the boiling point of ethers.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The electron withdrawing group (-NO2), withdraws electrons and disperses the negative charge. Therefore, -NO2 group stabilizes the phenoxide ion. Hence p-nitrophenol is more acidic than phenol.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The occurrence of intermolecular hydrogen bonds due to the H- bonds in alcohol compounds play a major role . With the increasing number of alkyl groups the molecular mass increases due to which the polar nature of these compounds get suppressed . Hence, the solubility factor of alcohols is indirectly proportional to the molecular mass.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The bond angle C—O—H in alcohols is less than tetrahedral angle because of the repulsion between the lone pairs present on the oxygen atom, which pushes the bond C—O—H closer. Therefore, C—O—H bond angle in alcohols is slightly less than the tetrahedral angle.
In ethers, the lone pairs on oxygen atoms are also present but the two bulky alkyl groups also have repulsive force which increases the C—O—C bond angle. Therefore, the C—O—C bond angle in ether is slightly greater than tetrahedral angle .
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
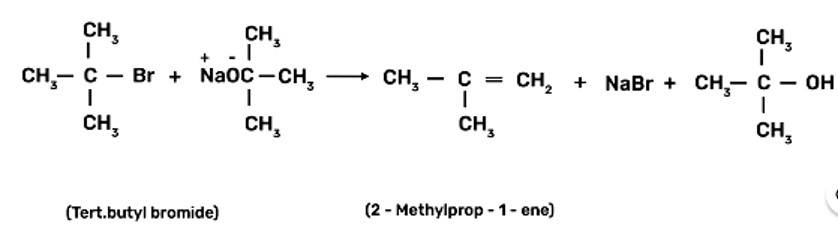
Ethers are prepared by Williamson synthesis by the reaction of alkyl halide with the stadium alkoxide. But this is not possible in case of di-tert-butyl ether. In case of preparation of di-tert-butyl ether, tert-butyl halide must react with sodium tert-butoxide. Alkoxides are nucleophiles but they are strong bases as well, due to which elimination is favoured instead of substitution and leads to the formation of 2-methylprop-1-ene.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In phenol, the electron pairs on oxygen atom of -OH group are in conjugation (or resonance) with phenyl ring which decreases the polarity of the C-OH bond whereas in methanol the polarity of C-OH bond is more due to electron releasing group of -CH3.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Phenoxide ion is more reactive than phenol to electrophilic aromatic substitution reaction due to the more in electron density of phenoxide ion and hence, phenoxide ion easily undergoes the electrophilic substitution reaction than phenol by the weak CO2 electrophile.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The nitration of aromatic compounds occurs by using HSO4 and HNO3 which leads to the formation of NO2+ (nitronium ion) electrophile.
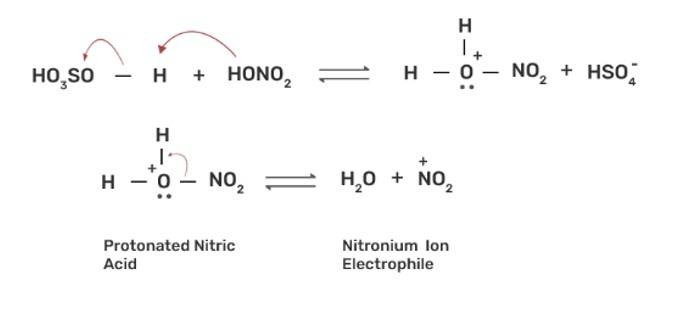
Due to the formation of NO2+ electrophile, we can say that nitration is an example of aromatic electrophilic substitution. The electron releasing group on the benzene ring increases the rate of nitration and vice-versa.
Phenol is more easily nitrated than benzene as the hydroxyl (-OH) group on the benzene ring is an electron releasing group which increases the electron density at the ortho and para position due to +R effect of -
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
