Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen
Get insights from 111 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
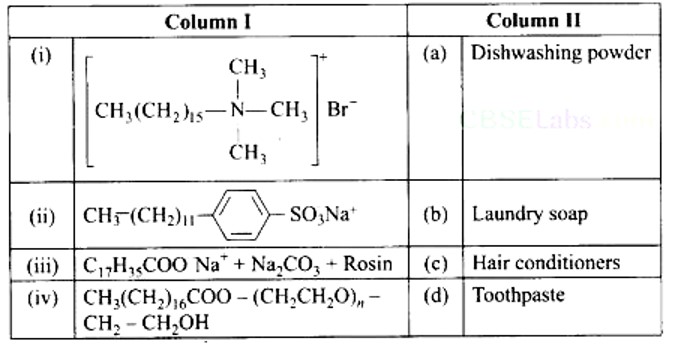
(i) → (c) (ii) → (d) (iii) → (b) (iv) → (a)
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
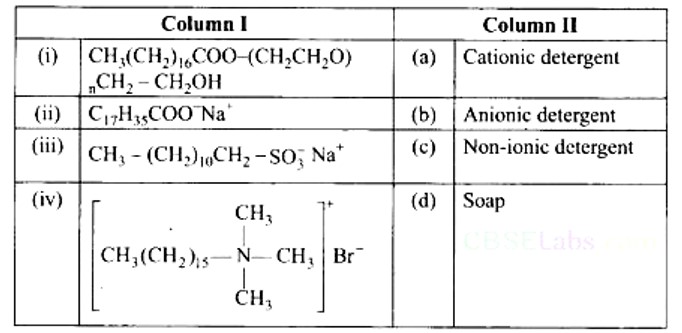
(i) → (c) (ii) → (d) (iii) → (b) (iv) → (a)
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
New answer posted
6 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (c) (ii) → (d) (iii) → (a) (iv) → (b)
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
If the bond formed between an enzyme and an inhibitor is a strong covalent bond and cannot be broken easily, then the enzyme is blocked permanently, The body then degrades the enzyme-inhibitor complex and synthesises the new enzyme.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Receptor proteins are embedded in cell membranes in such a way that their small part possessing active sites projects out of the surface of the membrane and opens on the outside region of the cell membrane.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
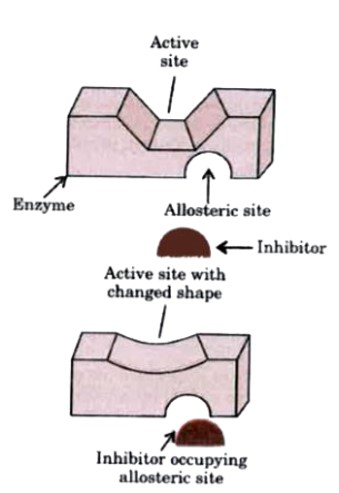
Some drugs do not bind to the enzyme's active site. These bind to a different site of enzyme which is called allosteric site. This bonding of inhibitor at allosteric site changes the shape of the active site in such a way that substrate cannot recognise it. As a result, the affinity of the substrate for the active site is reduced.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Sodium salts of benzoic acid, sorbic acid and propanoic acid, etc., are used as food preservatives.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Since aspartame is unstable and decomposes at cooking temperature, therefore, it is used as a sweetening agent in cold foods and soft drinks.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
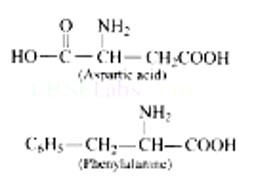
Aspartic acid and phenylalanine are two a-amino acids which form the methyl ester of dipeptide and named as aspartame (an artificial sweetener) which is 100 times more sweet than cane sugar.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers