Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen
Get insights from 111 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Sixteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Soaps are biodegradable. The detergents are quite stable and are non- biodegradable because of branching in hydrocarbon chain hence cause water pollution. Therefore, it is safer to use soap from the environmental point of view.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Straight chain hydrocarbons in synthetic detergent leads to easy biodegrad-ability because the branched chain hydrocarbon tail is a source of pollution. Therefore, lesser the branching more is the biodegradability.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
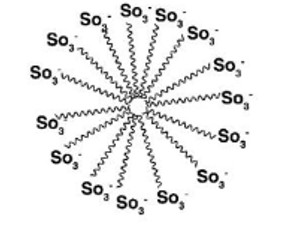
Micelle formation of the detergent can be shown as:
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Non-ionic detergents such as polyethylene glycol stearate are used as dishwashing soaps.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Cationic detergents such as cetyltrimethyl ammonium bromide are used in hair shampoos and hair conditioners.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Anionic detergents such as cetyltrimethyl ammonium bromide are used in hair shampoos and hair conditioners.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Detergents (which are not biodegradable) persist in water even after sewage treatment and that causes foaming in river water.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Acid-base titration can be used to determine the excess amount of alkali in soap. The excess alkali left after hydrolysis of oils or fats can be the source of alkalinity in soap.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Soft soaps are potassium salts of fatty acids (such as palmitic acid, stearic acid and oleic acid).
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Antihistamines are the drugs which control the allergy effects produced by histamines. Antacids are the substances which neutralise gastric acidity. Antihistamine do not affect the secretion of acid in stomach and the reason is that both antiallergic and antacid drugs work on different receptors.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers