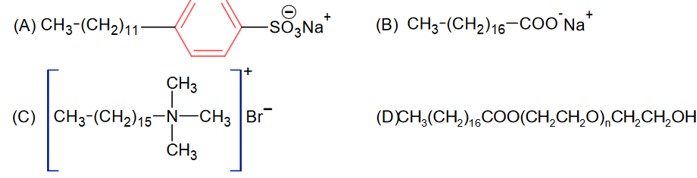Chemistry NCERT Exemplar Solutions Class 12th Chapter Two
Get insights from 140 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Two, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Two
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Water has only two lone pair and XeF4 has two lone pair electron in opposite plane of the central atom.
New answer posted
5 months agoContributor-Level 10
After removal A = 4
After removal B = 4 – 1 = 4 (only two atoms are removed)
Final formula of the compound = A3B4
New answer posted
5 months agoContributor-Level 10
CH3 – (CH2)16 – COONa is the sodium salt of fatty acid used in soap is not synthetic detergent.
New answer posted
5 months agoContributor-Level 10
p- toluenesulphonyl chloride
Only gives soluble product with alkali for 1° amine and for 2°- amine it gives insoluble product.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers