Chemistry NCERT Exemplar Solutions Class 12th Chapter Two
Get insights from 140 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Two, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Two
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Be does not perform in flame colouration due to highly ionization energy.
New answer posted
5 months agoContributor-Level 10
In sulphide ore, depressants selectively prevent impurity from coming to the fourth.
New answer posted
5 months agoContributor-Level 10
Invertase Cane sugar to glucose and fructose
Zymase Glucose to ethanol
Diastase Starch to Maltose
Maltase Maltose to glucose
New answer posted
5 months agoContributor-Level 10
In 4d orbital, n = 4 and
Radial nodes =
Radial nodes = 4 – 2 – 1 = 1
And angular nodes,
New answer posted
5 months agoContributor-Level 10
(A) Cr = [Ar]3d54s1
(B) m =
(C) According to Aufbau principle, orbital are filled in order of their increasing energies.
(D) Total nodes = n – 1
New answer posted
5 months agoContributor-Level 10
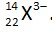
Number of electrons = 25
% of extra electrons =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers