Chemistry Solutions
Get insights from 89 questions on Chemistry Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
0.5 % KCl solution has molality (m) =
1 - a a a
And I =
1.976 = 1 + a
% = 97.6%
the nearest 98.
New answer posted
4 months agoContributor-Level 10
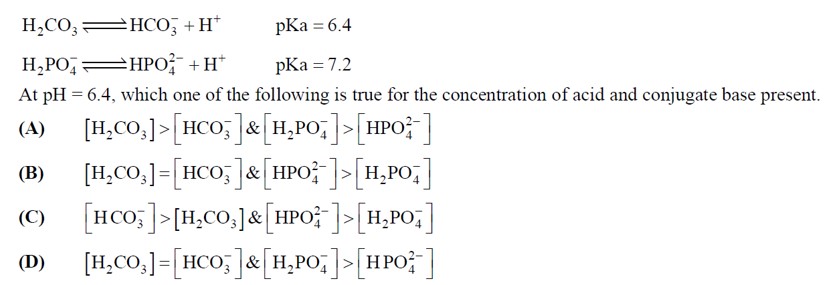
At pH = 6.4
As in case of H2CO3,
pH = pKa it will be only when
[weak acid] = [conjugate base].
In case of 2
H2PO-4 |HPO2-4
New answer posted
4 months agoContributor-Level 10
∴ % of S in the compound
= (32/233) * (mass of BaSO? / mass of compound) * 100 = (32 * 0.35 * 100) / (233 * 0.25) = 19.227 ≈ 19.23
New answer posted
4 months agoContributor-Level 9
1000 ml solution contains 0.02 milli mole (mm)
500 ml solution contains 0.02 m.m
Solution made 1000 ml with H2O
m.m in final solution = 0.01 mm
Solution (A) + 0.01 m. m H2SO4
= 0.01 + 0.01
= 0.02 m.m
= 0.00002 * 103 mm
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers