Chemistry Solutions
Get insights from 89 questions on Chemistry Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Π = iCRT = I (n/V)RT
Πfinal = (Π? V? + Π? V? )/ (V? + V? )
Πfinal = [ (0.1 * 1) + (0.2 * 2)]/3
= (0.1 + 0.4)/3 = 0.5/3 = (500/3) * 10? ³ atm
so X = 167
New answer posted
4 months agoContributor-Level 10
Equivalent of solute = 0.1 * 0.1
Mole of solute (Na? CO? ·xH? O) = [0.1 * 0.1]/2
Mass of Na? CO? ·xH? O = ( [0.1 * 0.1]/2) * [106 + 18x] = 1.43
⇒ 106 + 18x = 286
18x = 180
x = 10
New answer posted
4 months agoContributor-Level 9
P? = X? (P? – P? ) + P?
ATQ
550 = 1/4 (P? – P? ) + P?
2200 = P? – P? + 4P?
560 = 1/5 (P? – P? ) + P?
2200 = P? – P? + 5P?
P? + 3P? = 2200
P? + 4P? = 2800
P? = 600
P? = 400 mmHg
New answer posted
4 months agoContributor-Level 10
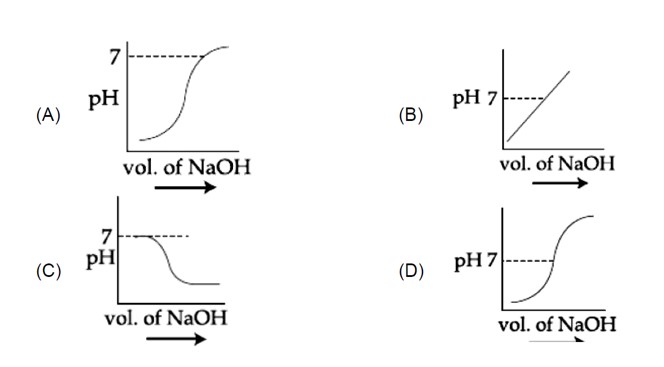
At equivalence point is 7 and increases with addition of so correct graph is (1).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers