Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
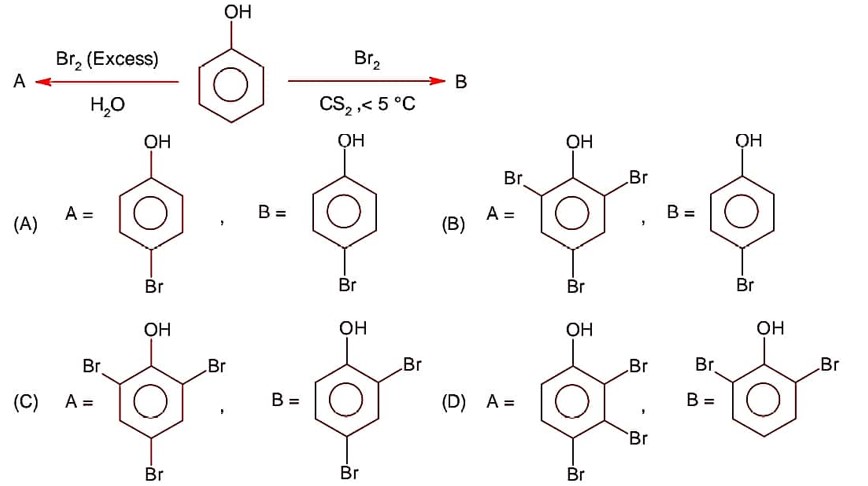
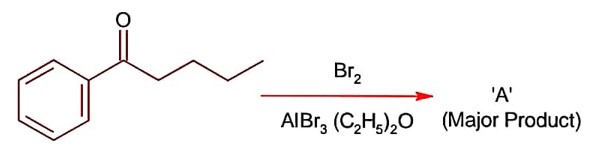
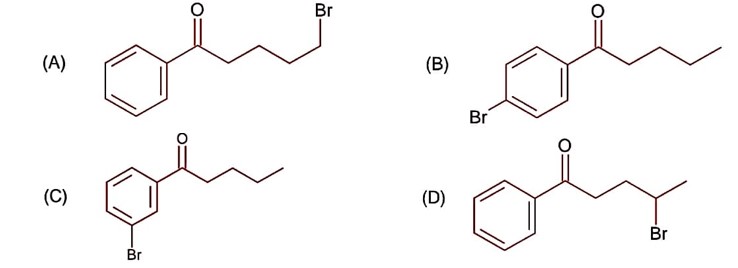
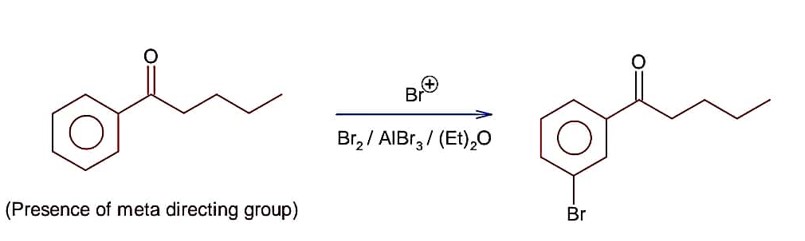
CS2 is non-polar, non-ionisable solvent and produce only para substituted compound due to the production of less Br+ ion, while H2O itself a polar solvent and produce sufficient concentration of Br+ to attack on ortho and para position.
New answer posted
5 months agoContributor-Level 10
Bakelite formed by the condensation reaction of phenol with formaldehyde and it is a thermosetting polymer/ plastics.
New answer posted
5 months agoContributor-Level 10
Electronic configuration of
In anion total 10 e- resides in Bonding Molecular Orbitals and 7e- resides in anti-Bonding Molecular orbitals. Hence bond order
It is paramagnetic due to one unpaired electron.
New answer posted
5 months agoContributor-Level 10
Heavy water (D2O) is used to study reaction mechanism. O-H bond energy is less than O-D, hence it will produce fast reaction than O-D. Here assertion is true but Reason is false.
New answer posted
5 months agoContributor-Level 10
shows cis and trans geometrical isomerism.
shows cis and trans geometrical isomerism.
can not show geometrical isomerism.
New answer posted
5 months agoContributor-Level 10
When AgNO3 added to Kl solution, it form precipitate of Agl (s) which absorb I- ions from Kl and colloidal particles become negatively charged.
New answer posted
5 months agoContributor-Level 10
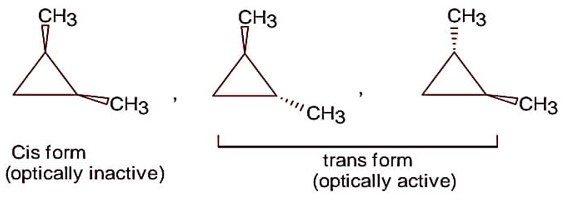
Following are the possible isomers of 1, 2-dimethyl cyclopropane
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers