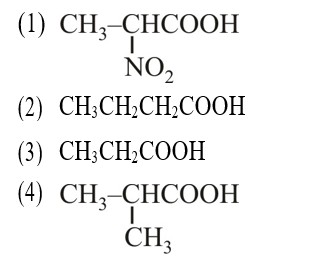Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
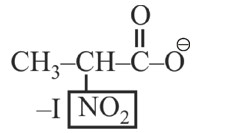
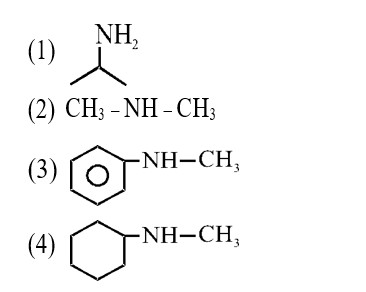
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
New answer posted
4 months agoContributor-Level 10
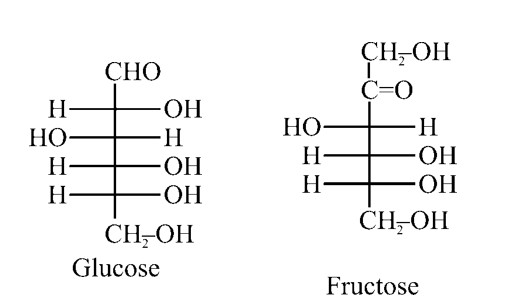
Glucose and fructose are functional group isomers of each other
New answer posted
4 months agoContributor-Level 10
[CrF6]–4
⇒ Cr+2 → 3d4
F– → WFL → No pairing so unpaired e– = 4
(b) [MnF6]–4
Mn+2 → 3d5
F– → WFL → No pairing unpaired e– = 5
(c) [Cr (CN)6]–4 ⇒ Cr+2 ⇒ d4 CN– → SFL
→ unpaired e– = 2
(d) [Mn (CN)6]–4 ⇒ 3d5,
unpaired e– = 1
New answer posted
4 months agoContributor-Level 10
[V (CO)6]
EAN = 23 – 0 + 2 * 6
= 23 + 12
= 35 ≠ 36
⇒ so it does not obey EAN rule.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers