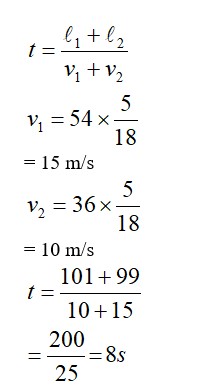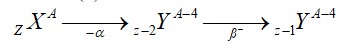Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Acidic nature of hydrides increases down the group in p-block
so H2Te will be most acidic among given options.
New answer posted
4 months agoContributor-Level 10
A jump is seen after 2nd Ip so Ve– = 2
hence configuration would be ns2
New answer posted
4 months agoContributor-Level 10
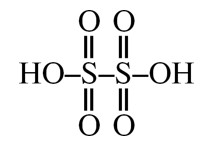
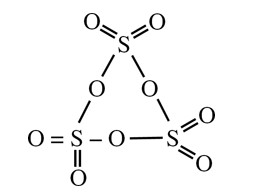
SO3 (s) exist as cyclic trimer ⇒ S3O9
→ sp3, so all bond π–bonds would be of pπ–dπ so pπ–pπ bond = 0
pπ–dπ bonds = 6
New answer posted
4 months agoContributor-Level 10
Photodiode in reverse bias mode is used as intensity measuring device.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers