Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
An ideal gas is a theoretical or hypothetical gas that obeys the ideal gas law perfectly. That is, PV=nRT.
New answer posted
5 months agoContributor-Level 10
Vernier calipers are instruments for making accurate linear measurements. They are commonly used to measure the diameter of a sphere or the depth of a hole. Its precision comes from a sliding Vernier scale that runs along a fixed main scale.
New answer posted
5 months agoContributor-Level 10
There are two types of errors in measurements. One is a systematic error, which comes from a fault in the measuring device. The other is a random error in measurement, which is caused by a sudden change in the environment.
New answer posted
5 months agoContributor-Level 10
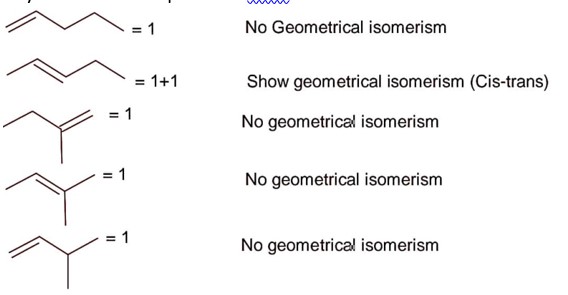
The circle not interesting either axes
(i) (ii) -> c > 100 .(iii)
Point of intersection between x – 2y – 4 = 0 & 2x – y – 5 = 0 is (2 - 1) lies inside the circle.
-> 100 < c < 156
New answer posted
5 months agoContributor-Level 10
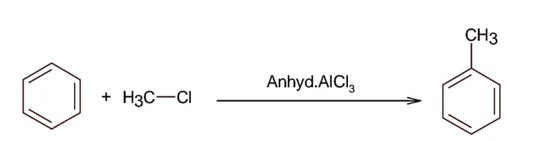
Theoretically; 1 mol benzene gives 1 mol toluene
Moles of benzene =
Moles of toluene (Theoretical) =
Mole of toluene (observed) =
% yield =
= 78%
New answer posted
5 months agoContributor-Level 10
Kp = 47.9 T = 288 K
KC =? R = 0.083 L bar/K mol
Using
Here ;
49.7 = KC (0.083 * 288)1
KC = 2
New answer posted
5 months agoContributor-Level 10
Sign conventions are important because all kinematic variables can be positive or negative. You must first choose your coordinate system and positive direction, then consistently apply signs. For example, if you are choosing upward as positive in free-fall problems, gravity becomes negative (a = -g), upward initial velocity is positive, and downward displacement is negative. The equations of motion work for any situation, as long as you substitute values with proper signs. Incorrect conventions lead to wrong answers.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers