Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Total wt = 4gm = WNaOH +
Let us suppose moles are 'm' for each.
4 = 40m + 106m
moles of NaOH and Na2CO3 each .
Mass of NaOH (x gm) =
New answer posted
5 months agoContributor-Level 10
for AgCl ppt1,
For Ag2CrO4ppt2,
Being lower concentration of [Ag+] in case of AgCl, it will precipitate first.
New answer posted
5 months agoContributor-Level 10
Regarding industrial consumption of H2 manufacturing of NH3 is the largest applications.
New answer posted
5 months agoContributor-Level 10
B (OH)3 is orthoboric acid having acidic nature, while Al (OH)3 has amphoteric nature that is acidic and as well as basic
New answer posted
5 months agoContributor-Level 10
B (OH)3 is orthoboric acid having acidic nature, while Al (OH)3 has amphoteric nature that is acidic and as well as basic
New answer posted
5 months agoContributor-Level 10
Nowdays liquid CO2 used as washing clothes, because Cl2 and C2H2Cl4 are toxic
New answer posted
5 months agoContributor-Level 10
An alkali metal oxide of formula MO2 is super oxide which is formed by K and Rb. KO2 has pale yellow colour and paramagnetic nature, while alkaline earth metal oxides formula MO2 are peroxide and diamagnetic nature.
New answer posted
5 months agoContributor-Level 10
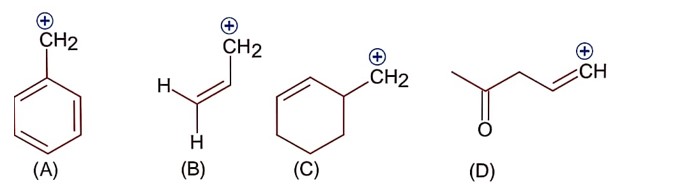
Only (A) and (B) structures have resonance effect. In (A) benzyllic resonance and in (B) allylic resonance of carbocation.
New answer posted
5 months agoContributor-Level 10
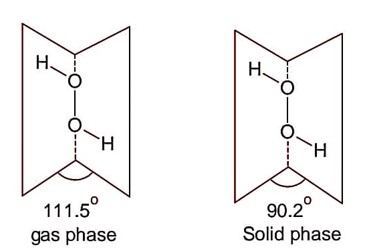
Following are the dihedral angles of H2O2 in gas phase and solid (Reverse with respect to question).
New answer posted
5 months agoContributor-Level 10
Given electronic configuration is for Ga and in 5th period diagonally situated element is Sn with respect to Ga. Hence electronic configuration of Sn is [Kr]4d105s25p2.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers