Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
S = {1, 2, 3, 4, 5, 6, 9}
Elements of type 3n -> 3, 6, 9
Type 3n + 1 ->1, 4
3n + 2 -> 2, 5
Number of subset of S containing one element which are not divisible by
Number of subset of S containing 3 elements whose sum is not divisible by
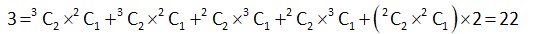
Number of subset containing 4 elements whose sum is not divisible by 3
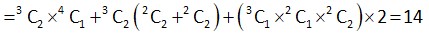
Number of subset of S containing 6 elements = 4
Hence total subset = 80
New answer posted
5 months agoContributor-Level 9
B. O. D. value < 5 ppm for clean water and B.O.D value of polluted water
New answer posted
5 months agoContributor-Level 10
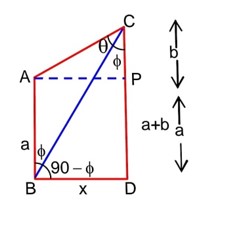
Given let α, β be the roots of the equation x2 + bx + c = 0
So,
Also x2 + bx + c = (x - α) (x - β)
=
New answer posted
5 months agoContributor-Level 9
Portland cement contains
Dicalcium silicate = 26%
Tricalcium silicate = 51%
Tricalcium aluminate = 11%
Hence major percentage is of tricalcium silicate
New answer posted
5 months agoContributor-Level 10
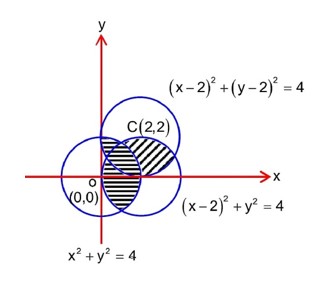
number of elements in
(0, 0) (1, 0) (1, 1) (1, -1) (2, 0)
Similarly number of elements in
(2, 0) (2, 2) (1, 1) (2, 1) (3, 1)
Hence number of relation from
->P = 25
New answer posted
5 months agoContributor-Level 10
So,
=> z is purely imaginary
i.e. x = 0 if z = x + iy
so, z = iy
=> S is a straight line in complex plane
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers