Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
High purity (> 99.95%) H2 gas is obtained by electrolysis of warm aqueous Ba (OH)2 solution using Ni electrode.
New answer posted
5 months agoContributor-Level 10
Ionization enthalpy: B < Be < O < N
IE of N > O [Due to half-filled configuration]
IE of Be > B [Due to penetration effect]
New answer posted
5 months agoContributor-Level 10
Z distribution and Chi-Squared are some of the most popular distribution patterns of probability, and it is vital to recognise the variations between them and when to use the distribution pattern. A Z table is of no use when the operation revolves around a smaller sample size. On the other hand, the distribution of a sum of independent regular k squares in standard normal variables is the chi-square distribution of k degrees of freedom. The tests are used for the independence of two variables in an incident table and to assess the observable data for
New answer posted
5 months agoContributor-Level 10
Five types of sample statistics include sample mean, sample variance, sample standard deviation, sample proportion.
- Sample mean is the average of all data points in a sample. It is calculated by summing all values in sample and then dividing by number of observations.
- The sample variance measures the dispersion and spread of data points in sample. It indicates the average of squared differences from sample mean.
- Sample standard deviation is the square root of the sample variance that provides a measure of dispersion in same units as data.
- The sample proportion is fraction of the sample that has a certain attribute or characteristics. This
New answer posted
5 months agoContributor-Level 10
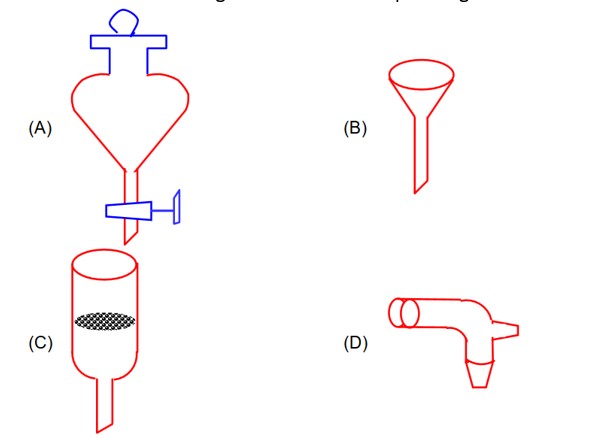
Funnel of option A is useful for the separation of liquid – liquid mixture of immiscible form.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers