Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Speed of sound wave in a medium v
Clearly when temperature changes speed also changes
As v= as frequency remains constant and speed changes so wavelength changes
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Due to presence of moisture density of air decreases.
As we know speed of sound in air v=
So v
v2/v1=
2 (moist air)< 1 (dry air)
So v2>v1
So speed of sound wave increases with increase in humidity
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(c) Let frequency of the two will be and it is same in both medium
So v/ = v'/
= v' /v
= 2v /v=2
Where and ' and v and v' are wavelength and speeds of first and second medium respectively
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b) Water waves produced by a motorboat are both longitudinal and transverse because it produce both lateral and transverse wave In the medium.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Let there are n number of loops in the string
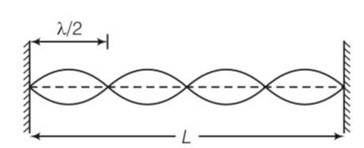
length corresponding each loop is
So we can write L=
v/ =2L/n
so frequency v=
v
so
= 1:2:3:4
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
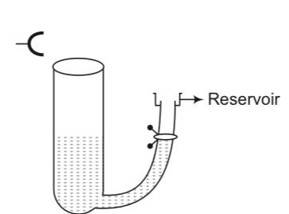
consider the frequency of tuning fork= 512Hz
(a) L=
V= = 512
= 348.16m/s
(b) we know that v
=
Vo=
(c) Resonance will be observed at 17cm length of air column only intensity of sound heard may be greater due to more complete reflection of the sound waves at the mercury surface because mercury is more denser than water.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
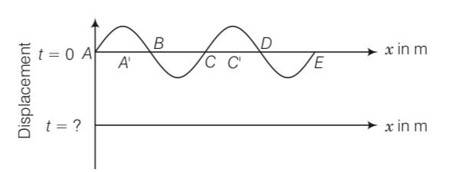
Given frequency of the wave v=256Hz
T=
(a) time taken to pass through mean position is
t=T/4=1/40 =3.9 9.8 10-4s
(b) nodes are A, B, C, D, E (having zero displacement)
antinodes are A' and C' (having maximum displacement)
(c) it is clear from diagram A' and C' are forming antinodes have separation= v/v'=360/256=1.41m
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
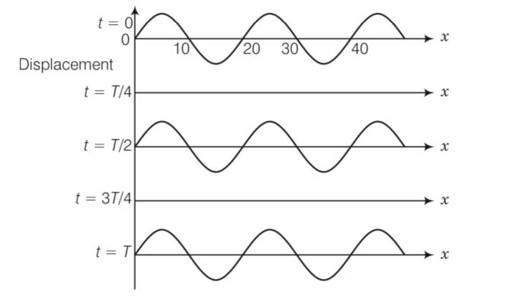
We have to observe the displacement and position of different points, then accordingly nature of two waves decided
Points on positions x= 10,20,30,40 never move, always at mean position with respect to time . these are forming nodes which forms a stationary wave
Distance between two successive nodes =
node to node distance)
=2 (20-10)
= 2 (10)=20cm
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
As the source is moving towards the observer hence apparent frequency observed is more than the natural frequency
Frequency of whistle
Speed of train = vt= 10m/s
Velocity of sound in air =v= 330m/s
Apparent frequency when source is moving vapp= (v/v-vt)v
= (
=
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
length of pipe
L=20cm =20
=3
Hence 3rd harmonic node of the pipe is resonantly excited by the source of the given frequency.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers