Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
(a) The element V has the highest first ionization enthalpy (? iH1) and positive electron gain enthalpy (? egH) and hence it is the least reactive element. Since inert gases have positive? egH, therefore, the element-V must be an inert gas. The values of? iH1, ? iH2 and? egH match that of He.
(b) The element II which has the least first ionization enthalpy (? iH1) and a low negative electron gain enthalpy (? egH) is the most reactive metal. The values of ? iH1, ? iH2 and? egH match that of K (potassium).
(c) The element III which has high fi
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, b, d, e) a) clearly every particle at x will have amplitude =asinkx=fixed
b) for mean position =0
coswt=0
wt= (2n-1)
hence for a fixed of n all particles are having same value of time t= (2n-1)
c) amplitude of all the particles are asinkx which is different for different particles at different values of x
d) the energy is a stationary wave is confined between two nodes.
e) particles at different nodes are always at rest.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, b) vo=400Hz, v=340m/s
Vm=10m/s
(a) as both source and observer are stationary, hence frequency observed will be same as natural frequency vo=400Hz
(b) the speed of sound v=v+vw= 340+10=350m/s
(c) there will be on effect on frequency because there is no relative motion between source and observer hence c, d are incorrect.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a, b, d) As we know by y (x, t) = 0.06 sin (2πx/3) cos (120πt)
By comparing the equation with general equation N denotes nodes and A denotes antinodes.
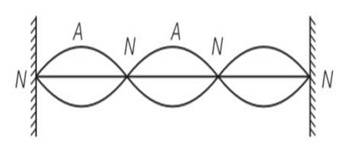
(a) Clearly frequency is common for all the points
(b) Consider all the particles between two nodes they are having same phase at given time
(c) But are having different amplitude of 0.06sin (2 ) and because of different amplitudes they are having different energies.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers







