Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(i) dimension of h = [ML2T-1]
And c= [LT-1] and dimension of G= [M-1L3T-2]
Let m= kcxhyGz
[ML0T0]= [LT-1]x [ML2T-1]y [M-1L3T-2]z
= [My-zLx+2y+3zT-x-y-2z]
y-z=1
x+2y+3z=0
-x-y-2z=0
On solving these equation we got x= ½ y= ½ and z= -½
So formula will coming out from this is m=k
(ii) L=kcxhyGz
ao [M0LT0]= [LT-1]x [ML2T-1]y [M-1L3T-2]z
= [My-zLx+2y+3zT-x-y-2z]
y-z=0
x+2y+3z=1
-x-y-2z=0
After solving we get x= -3/2 y=1/2 and z=1/2
We got the formula is L=k
(iii)T= kcxhyGz
[M0L0T]= [LT-1]x [ML2T-1]y [M-1L3T-2]z
= [My-zLx+2y+3zT-x-y-2z]
On comparing powers we got x= -5/2 y=1/2 an
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Dimensions of energy is E= [ML2T-2]
Mass m= [M]
Dimension of E= [ML2T-2]
Dimensions of L= [ML-2T-1]
Dimensions of G= [M-1L3T-2]
By using these values [P]= [ML2T-2] 2 -2
= [M1+2-5+2L2+4-6T-2-2+4]
= [M0L0T0]
After we know that P is dimensionless quantity
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
As we know that X= a2 b3 c5/2 d-2
Maximum percentage error in X is
=
Mean absolute error in X= rounding off to significant value.
And calculated value would be 2.8 rounding off upto two digits.
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Rate of flow is equal to V=
Dimensions of V or LHS= volume/time=L3/T= [L3T-1]
Dimensions of P= [ML-1T-2]
Dimensions of = [ML-1T-1]
Dimensions of L= [L]
Dimensions of r= [L]
Dimensions of RHS=
So they are in equal in dimensions.
So equation is correct dimensionally.
New answer posted
6 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Energy E= [ML2T-2]
Let M1, L1 and T1 and M2, L2 and T2 are fundamental quantities for two units
M1=1kg and L1=1m and T1=1s
M2= α kg, L2= β m and T2= γ s
And n1u1=n2u2
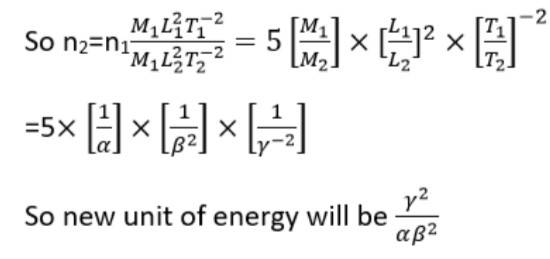
New answer posted
6 months agoContributor-Level 10
According to the de Broglie equation, the particles like electrons have wave-like properties. The quantum mechanical model foundation is laid by this concept and it also explains the stability of electron orbits using wave behavior.
New answer posted
6 months agoContributor-Level 10
The hydrogen atom can be accurately explained by Bohr's model. However, it does not account for shielding effects, electron-electron interactions, and the wave nature of electrons for multi-electron atoms.
New answer posted
6 months agoContributor-Level 10
Quantum numbers describe the unique position and energy of an electron in an atom. These are a set of four numbers and are important for predicting chemical behavior and understanding the distribution of electrons in orbitals.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Time period of simple pendulum T=2s
For simple pendulum T= where l is length and g = acceleration due to gravity.
Te=2
On the surface of the moon Tm= 2
=
Te=Tm to maintain the second's pendulum time period
1= …………….1
But the acceleration due to gravity at moon is 1/6 of the acceleration due to gravity at earth,
gm=
squaring equation 1 and putting this value
1=
lm=1/6le = 1/6 m
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
