Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
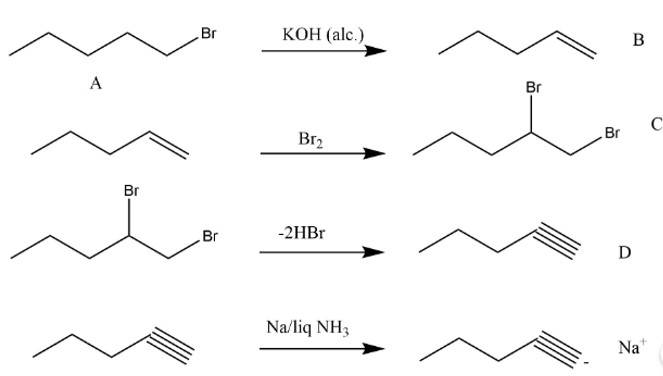
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
27. (a) The magnitude of the enthalpy change depends on the strength of the intermolecular interactions in the substance undergoing the phase transformations. For example, the strong hydrogen bonds between water molecules hold them tightly in liquid phase. For an organic liquid, such as acetone, the intermolecular dipole-dipole interactions are significantly weaker. Thus, it requires less heat to vaporise 1 mol of acetone than it does to vaporize 1 mol of water.
New answer posted
7 months agoContributor-Level 10
26. (b) A system at higher temperature has greater randomness in it than one at lower temperature. Thus, temperature is the measure of average chaotic motion of particles in the system.
New answer posted
7 months agoContributor-Level 10
25. (c) When a liquid crystallizes into solid or after freezing, the molecules attain an ordered state and therefore, entropy decreases.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
