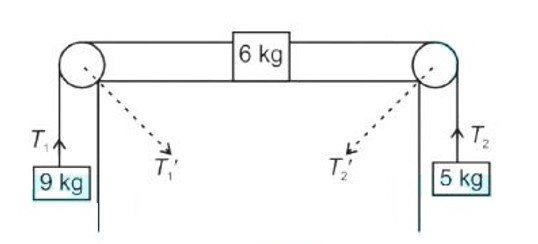
Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
In a number without decimal, trailing zeros are insignificant. If a number has a decimal, then preceding zeros are insignificant.
Hence, in 40.40 all zeros are significant
New answer posted
3 months agoContributor-Level 10
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
New answer posted
3 months agoContributor-Level 10
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of delocalized electrons, metallic bonds get created.
New answer posted
3 months agoContributor-Level 9
Rate of burning of fuel
Forcev =
New answer posted
3 months agoContributor-Level 10
The chemical bonds can be primary or strong bonds such as ionic, covalent, and metallic bonds. It can also be the secondary or weak bonds like hydrogen bonding, London dispersion force, and dipole interactions.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers