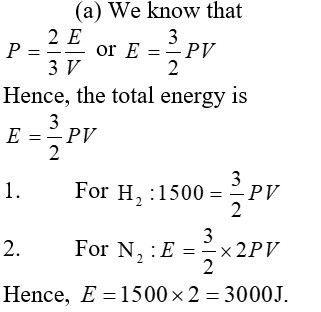Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
On increasing pressure, equilibrium moves in that direction where number of gaseous moles decreases.
New answer posted
3 months agoContributor-Level 10
Total kinetic energy associated with 'n' moles of monoatomic gas is =
New answer posted
3 months agoContributor-Level 10
NH3 can be liquefied most easily due to presence of strong intermolecular H-bonding among NH3 molecules.
New answer posted
3 months agoContributor-Level 10
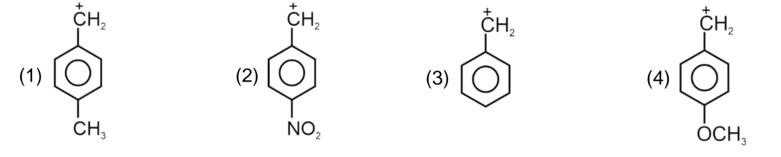
Electron withdrawing group decreases the stability of carbocation.
Correct order of stability of carbocation is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers