Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
H2O2 decomposes slowly on exposure to light.
2H2O2→ 2H2O+2O2
In daily life it is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(i) Oxidising action in acidic medium:
2Fe2+ (aq)+2H+ (aq)+H2O2 (aq) → 2Fe3+ (aq)+2H2O (l)
PbS (s)+4H2O2 (aq) → PbSO4 (s)+4H2O (l)
(ii) Reducing action in acidic medium:
2MnO4-+6H++5H2O2 → 2Mn2++8H2O+5O2
HOCl+H2O2 → H3O++Cl-+H2O
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
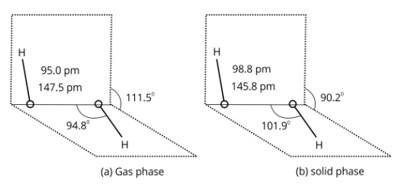
H2O2 structure in gas phase, dihedral angle is 111.50. H2O2 structure in solid phase at 110 k, dihedral angle is 90.20.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Heavy water can be used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It can be prepared by exhaustive electrolysis of water or as a by-product in some fertilizer industries.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Hydrogen economy (Hydrogen as fuel):
(i) The electricity cannot be stored in automobiles. It is not possible to store and transport nuclear energy. Hydrogen is an alternative source of energy and hence called the 'hydrogen economy". Hydrogen has some advantages as fuel.
(ii) Available in abundance in combined form as water.
(iii) On combustion produces H2O. Hence, pollution free.
(iv) H2O2 fuel cells give more power.
(v) Excellent reducing agent. Therefore, it can be used as a substitute of carbon in reduction for processes in industry.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar\
In sodium the last shell electron is in 3s1 after losing that electron, it can acquire the configuration of Ne. Whereas in hydrogen, the electron is in s-orbital.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Because of ionization enthalpy, hydrogen resembles more with halogens, is 520 kJ mol-1, F is 1680 kJ mol-1and that of His 1312 kJ mol-1. Like halogens, it forms a diatomic molecule, combining with elements to form hydrides and a large number of covalent compounds.
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
The equation can be written as-
Ap+x + Bq- y ? xAp+ (aq) + yBq-
S moles of A and B dissolves to give x S moles of Ap+ and y S moles of Bq-.
Ksp = [Ap+]x [Bq-]y = [x5]x [y5]y
= xx y y 5 x+y
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: option (iv)
If the volume is kept constant and an inert gas such as argon is added which does not take part in the reaction, the equilibrium remains undisturbed.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
