Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Due to difference in conductivity metals having high conductivity compared to wood. On touch with a finger heat from the surroundings flows faster to the finger from metals and so one feels the heat.
Similarly when one touches a cold metal the heat from the finger flows away to the surroundings faster.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
= oC-1
From next observation
From next observation
From next observation = so this value satisfies the equation.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
As diathermic walls allow exchange of heat energy between two systems and adiabatic walls do not, hence diathermic walls are used to make the bulb of a thermometer.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(a) Power radiated by stefan's law
P= 4 R2)T4
= 5.67
= 1.78 =1.8
(b) Energy available per second U= 1.8 = 18 16J/s
Actual energy required to evaporate water = 10%of 18
=1.8 J/s
Energy used per second to raise the temperature of m kg of water from 300C to 1000C and then into vapour at 1000C
=msw +mL= m
= 2.93
As per question , 25.53 = 1.8
m=
(c) Momentum per unit time
P= U/c= 2
Momentum per unit time
Area p= p/4
d=47.7N/m2
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Consider the diagram

Θ= θ1+ θ2/2
Let temperature varies linearly in the rod from its one end to other end, let θ be the temperature of the midpoint of the rod. At steady state
Rate of flow of heat,
dQ/dt =
where k is the coefficient of thermal conductivity of the rod
so θ1- θ= θ- θ2
θ= θ1+ θ2/2
L=L0 (1+ )
L= Lo (1+ )
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
By applying Pythagoras theorem in given figure
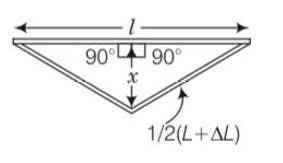
2
x=
=1/2
= ½
=1/2
As Increase in is very small so we neglect it
=1/2
By using this value in above in equation
x=1/2 = ½ L
=
= 5
= 5
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Decrease in temperature = 57-37= 200C
Coefficient of linear expansion = 1.7 oC
Bulk modulus for copper B = 140
Coefficient of cubical expansion = 3 = 5.1
Let initial volume of the cavity be V and its volume increases by due to increase in temperature.
Thermal stress produced = B
= B
= 140
= 1428 2
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
As difference in volume is constant
By considering the diagram
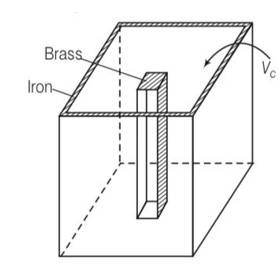
Let Vio , Vbo be the volume of iron and brass vessel at 00C
Vi,Vb be the volume of iron and brass vessel at 0C
be the coefficient of volume expansion of iron and brass.
Vio -Vbo= 100cc= Vi-Vb
Vi =Vio(1+ i )
Vb =Vbo(1+ b )
Vi-Vb = (Vio -Vbo)+
Since Vi-Vb= constant
Vio i= Vbo
Using above equations
Vbo = 144.9cc
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
As liron-lbrass =10cm= constant at all temperature
Let lo be the length of temperature at 00C and l be the length after change in temperature
liron-lbrass =10cm
liron (1+ )-lbrass (1+ )=10cm
Iiron iron= Ibrass brass
=1.8/1.2=3/2
Lbrass=20cm and liron=30cm
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(b), (c) Streamline flow is more likely for liquids having low density. We know that greater the coefficient of viscosity of a liquid more will be velocity gradient hence each line of flow can be easily differentiated. Also higher the coefficient of viscosity lower will be Reynolds number, hence flow more like to be streamline.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers