Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(a) F= V
F
Hence buoyancy will be less in water at 00C than that in water at 40C.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
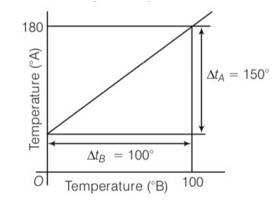
(b) Lowest point for scale A is 300 and lowest point for scale B is 00. Highest point for the scale A is 1800 and for scale B is 1000
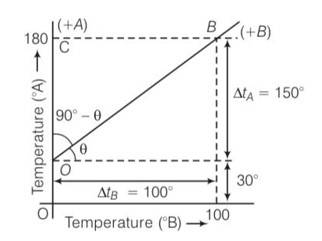
,LFP= lower fixed point , UFP= upper fixed point
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
L = angular momentum = Iw = constant
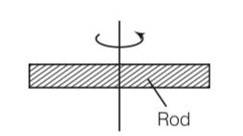
I1w1=I2w2
Due to expansion of the rod I2>I1
w2
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
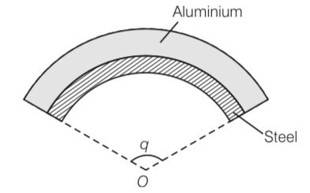
(d) As , aluminium will expand more . so it would have larger radius of curvature. So aluminium will expand and will outside region.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The first option kept water warmer because according to newton's law of cooling the rate of loss of heat is directly proportional to the difference of temperature of the body and the surroundings and in the first case temperature difference is less. So rate of loss of heat will be less.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Mass of water m =100
Change in temperature
Specific heat of water Sw= 1cal/g C
Latent heat of fusion of water Lfusion= 80cal/g
Heat required to bring water in super cooling from -10 to 0
Q= ms
Let m gram of ice be melted Q= mL
m=Q/L=1000/80= 12.5g
New answer posted
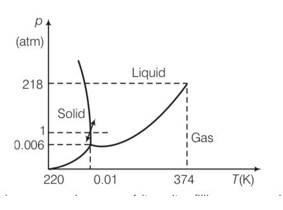
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Increasing pressure at 00C and 1 atm takes ice into liquid state and decreasing pressure in liquid state at 00C and 1 atm takes to ice state.
When crushed ice is squeezed, some of it melts, filling up the gap between ice flakes upon releasing pressure. This water freezes, binding all ice flakes and making the ball more stable.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Let the mass and length of a uniform rod be M and l respectively.
Moment of inertia of the rod about its perpendicular bisector I= ml2/12
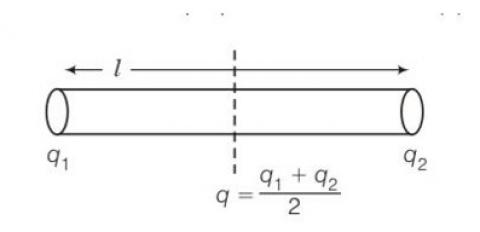
Increase in length of the rod when temperature is increased by T is given by
So new moment of inertia of the rod = =
As change in length is very small therefore neglecting so we get
I'= = l+mI
Increase in moment of inertia =
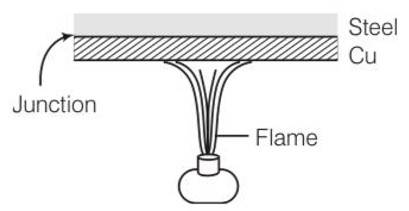
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
As copper is a good conductor of heat as compared to steel. The steel utensils with copper bottom absorbs heat more quickly than steel and give it to food in utensils. So, food heated uniformly and quickly.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
According to the formula
F=C=Q
= Q=-40C or 40F
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers