Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
Solubility of Sr (OH)2=19.23g/L
The molecular weight of Sr (OH)2 is 87.6 + 2 (17)=121.6
Then, concentration of Sr (OH)2=19.23 /121.63M=0.1581M
Sr (OH)2 (aq)→Sr2+ (aq)+2 (OH−) (aq)
∴ [Sr2+]=0.1581M
[OH−]=2*0.1581M=0.3126M
Now, Kw= [OH−] [H+]
=> [H+] = 10−14 / 0.3126
=> [H+]=3.2*10−14
∴pH= 13.495
New answer posted
8 months agoContributor-Level 10
[KOH]= [K+]= [OH−]= (0.561*1000) / (56*200)? =0.050M
[H+]=Kw / [OH−]? =10−14 / 0.05? =2.0*10−13
pH=−log [H+]=−log (2.0*10−13)
=12.7
New answer posted
8 months agoContributor-Level 10
The hydrogen ion concentration in the given substances can be calculated by using the given relation: pH=−log [H+]
Hence, [H+] = 10−pH
Milk: [H+] = 10−6.8 = 1.58*10−7M
Black coffee: [H+] = 10−5.0 =1*10−5M
Tomato juice: [H+] = 10−4.2 =6.31*10−5M
Lemon juice: [H+]=10−2.2 = 6.31*10−3M
Egg white: [H+]=10−7.8=1.58*10−8M
New answer posted
8 months agoContributor-Level 10
a. Human muscle fluid 6.83
pH=6.83
pH=−log [H+]
∴6.83=−log [H+]
[H+]= 1.48 x 10−7M
b. Human stomach fluid, 1.2:
pH=1.2
1.2=−log [H+]
∴ [H+] = 0.063 M = 6.3 x 10-2 M
c. Human blood, 7.38:
pH=7.38=−log [H+]
∴ [H+]= 4.17 x 10−8M
d. Human saliva, 6.4:
pH=6.4
6.4=−log [H+]
[H+]= 3.98 x 10−7 M
New answer posted
8 months agoContributor-Level 10
Kb= 5.4*10−4
c= 0.02M
Then, α= (Kb /c)1/2
α= (5.4*10−4 / 2 x 10-2)1/2 =0.1643
(CH3)2NH+H2O ↔ (CH3)2NH+2+OH-
[ (CH3)2NH] = 0.02 – x ≈ 0.02
[ (CH3)2NH+2] = x
[OH-] = 0.1 + x
≈ 0.1
Now, Kb= [ (CH3)2NH+2] [OH−]/ [ (CH3)2NH] = (x * 0.1) / (0.025).
x = 1.08 x 10-4
% of dimethylamine ionised = (1.08 x 10-4) x (100 / 0.02) = 0.54%
New answer posted
8 months agoContributor-Level 10
pKa? =? logKa= 4.74
Ka? = 10? pKa =10?4.74 = 1.8*10?5
Let x be the degree of dissociation. The concentration of acetic acid solution, C = 0.05 M
The degree of dissociation,
x= (Ka / C)1/2? = (1.8*10?5 / 0.05)1/2 ? = 0.019
(a) The solution is also 0.01 M in HCl.
Let x M be the hydrogen ion concentration from ionization of acetic acid. The hydrogen ion concentration from ionization of HCl is 0.01 M. The total hydrogen ion concentration
[H+] = 0.01 + x
The acetate ion concentration is equal to the hydrogen ion concentration from ionization of acetic acid. This is also equal to the concentration of acetic acid that has dissociated.
[CH3?
New answer posted
8 months agoContributor-Level 10
Let c be the initial concentration of C6H5NH3+ and x be the degree of ionisation.
C6H5NH2 + H2O? C6H5NH3+ + OH-
c (1-x) cx cx
Kb = [C6H5NH3+] [ OH-] / [C6H5NH2]
= [cx] [cx] / [c (1 – x)]
Since x is very small and negligible 1 – x≈ 1
∴Kb= [cx] [cx] / [c] = cx2
=> x =
=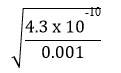
= 6.56 x 10-4
∴ [OH-] = cx = 0.001 x 6.56 x 10-4 = 6.56 x 10-7 M
[H+]= Kw / [OH-] = 10-14 / 6.56 x 10-7 = 1.52 x 10-8
pH= –log [H+] = –log1.52 x 1
New answer posted
8 months agoContributor-Level 10
pH = 9.95,
pOH = 14 – pH = 14 − 9.95 = 4.05
[OH−] = 10−pOH = 10−4.05 = 8.913 * 10−5
Codeine + H2? O? CodeineH+ + OH−
The ionization constant, Kb? = [CodeineH+] [OH−] / [codeine]?
= [ (8.913*10−5)* (8.913*10−5)] / 5*10−3
= 1.588*10−6.
pKb? = −log (1.588*10−6)
= 5.8
New answer posted
8 months agoContributor-Level 10
H+] = cα = 0.1 * 0.132 = 0.0132M
pH = −log [H+] = −log0.0132
= 1.88
The acid dissociation constant is
Ka? = cα2? / (1−α) = 0.1 * (0.132)2 / (1−0.132)
= 2.01*10−3.
pKa? = −logKa? = −log (2.01*10−3) ≈ 2.7
New answer posted
8 months agoContributor-Level 10
(a) For 2g of TlOH dissolved in water to give 2 L of solution:
[TlOH] = [OH−] = (2*1)? / (2*221) = (1 / 221)? M
pOH = −log [OH]− = −log (1/221)?
= 2.35
pH = 14 – pOH = 14 − 2.35 = 11.65
(b) For 0.3 g of Ca (OH)2? dissolved in water to give 500 mL of solution:
[OH−] = 2 [Ca (OH)2? ] = 2 (0.3*1000/500? ) = 1.2M
pOH = −log [OH−] = −log1.2 = 1.79
pH= 14−pOH=14−1.79
=12.21
(c) For 0.3 g of NaOH dissolved in water to give 200 mL of solution:
[OH−]= [NaOH] = 0.3*1000/200? = 1.5M
pOH= −log [OH−] = −log1.5 = 1.43
pH= 14 – pOH = 14 − 1.43
= 12.57
(d) For 1mL of 13.6 M HCl diluted w
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
