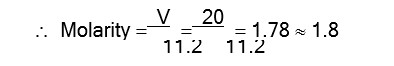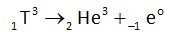Hydrogen
Get insights from 182 questions on Hydrogen, answered by students, alumni, and experts. You may also ask and answer any question you like about Hydrogen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
HF molecules are associated with strong intermolecular hydrogen bonding hence its boiling point is the highest
New answer posted
4 months agoContributor-Level 10
(-1) oxidation (0)
Here, I is reducing agent
behaves like oxidizing agent
New answer posted
4 months agoContributor-Level 10
Tritium is radioactive and it decays into He3 during emission of b-radiation
New answer posted
4 months agoContributor-Level 10
Due to H- bond in water, it has high melting point and melting point of other hydrides of the group are depending upon the molecular weight.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers