Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
3.28. The elements of Group I have only one electron in their respective valence shells and thus have a strong tendency to lose this electron. The tendency to lose electrons in turn, depends upon the ionization enthalpy. Since the ionization enthalpy decreases down the group therefore, the reactivity of group 1 elements increases in the order Li < Na < K < Rb < Cs. In contrast, the elements of group 17 have seven electrons in their respective valence shells and thus have strong tendency to accept one more electron to make stable configuration. So for group 17, the electron gain enthalpy and electronegativity decreases down the group and thus the reactivity also decreases.
New answer posted
8 months agoContributor-Level 10
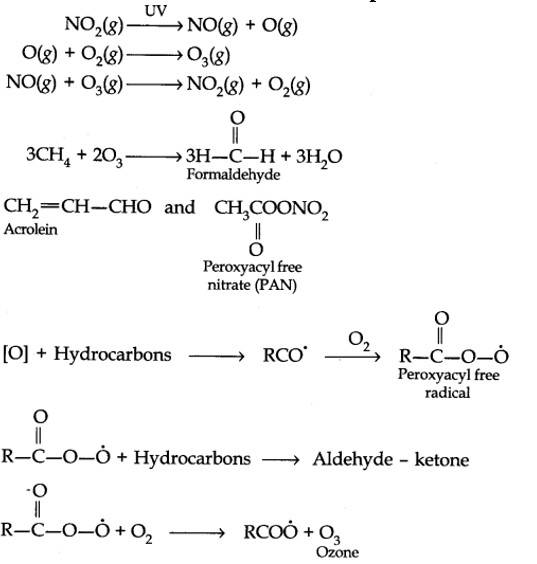
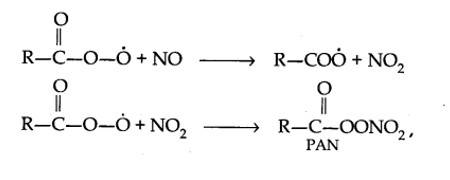
Harmful effects of photochemical smog: Photochemical smog causes serious health problems. Both ozone and PAN (peroxyacetyl nitrate) act as powerful eye irritants. Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing. Photochemical smog leads to cracking of rubber and extensive damage to plant life. It also causes corrosion of metals, stones, building materials, rubber and painted surfaces.
Control:
- Control of primary precursors of photochemical smog, such as NO2 and hydrocarbons, the secondary precursors such as ozone and PAN
New answer posted
8 months agoContributor-Level 10
3.27. (a) Element belonging to nitrogen family (group 15) e.g., nitrogen.
(b) Element belonging to alkaline earth family (group 2) e.g., magnesium.
(c) Element belonging to oxygen family (group 16) e.g., oxygen.
New answer posted
8 months agoContributor-Level 10
The word smog is a combination of smoke and fog. It is a type of air pollution that occurs in many cities throughout the world. Classical smog occurs in a cool, humid climate. It is also called reducing smog. Whereas photochemical smog (photo means light) occurs in warm and dry sunny climates. It has a high concentration of oxidising agents and therefore, it is also called oxidising smog.
New answer posted
8 months agoContributor-Level 10
This is mainly due to the large number of industries and power plants in the nearby areas. Acid rain has vapours of sulphuric acid dissolved in it. When it comes in contact with various statues or monuments, the acid reacts chemically with marble (calcium carbonate).
CaCO3 + H2SO4 à CaSO4 + H2O + CO2
As a result, the monument is being slowly disfigured, and the marble is getting discoloured and lustreless.
New answer posted
8 months agoContributor-Level 10
3.26.
Metals | Non-metals |
1. They have strong tendency to lose electrons to form cations. | 1. They have strong tendency to accept electrons to form anions. |
2. Metals are strong reducing agents. | 2. They are strong oxidising agents. |
3. Metals have low ionization enthalpies. | 3. Non-metals have high ionization enthalpies. |
4. They form basic oxides and ionic compounds. | 4. They form acidic oxides and covalent compounds. |
New answer posted
8 months agoContributor-Level 10
CO2 is mainly responsible for the greenhouse effect. Other greenhouse gases are methane, nitrous oxide, water vapours, CFCs (chlorofluorocarbons) and Ozone.
New answer posted
8 months agoContributor-Level 10
Carbon monoxide binds to haemoglobin to form carboxyhaemoglobin, which is about 300 times more stable than the oxygen-haemoglobin complex. In blood, when the concentration of carboxyhaemoglobin reaches about 3–4 per cent, the oxygen-carrying capacity of blood is greatly reduced. This oxygen deficiency results into headache, weak eyesight, nervousness and cardiovascular disorder. This is the reason why people are advised not to smoke. In pregnant women who have the habit of smoking the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies. On the other hand, CO2 does not combine with ha
New answer posted
8 months agoContributor-Level 10
Tropospheric pollution occurs due to the presence of undesirable solid or gaseous particles in the air. The following are the major gaseous and particulate pollutants present in the troposphere:
1. Gaseous air pollutants: These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
2. Particulate pollutants: These are dust, mist, fumes, smoke, smog etc.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers