Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
6.9 Let us assume
Body mass = m
Acceleration = a
According to Newton's 2nd law F = MA (constant)
We know a = dv/dt = constant. Hence dv = dt constant
On integrating, v = t + constant
The relation of power is given by P = F
We have
Hence,
=
=
Therefore P
New answer posted
8 months agoContributor-Level 10
6.8 (a) In elastic collision, the initial and final kinetic energy is equal. When the two balls collide, there is no conservation of kinetic energy; it gets converted into potential energy.
(b) The total linear momentum is conserved in an elastic collision.
(c) In case of inelastic condition in case (a), there will be loss of kinetic energy but in case of (b), the total linear momentum will be conserved in inelastic collision also.
(d) It is an elastic collision as the forces involves are conservative forces.
New answer posted
8 months agoContributor-Level 10
6.7 (a) False. The total momentum and energy is conserved, not the individual.
(b) False. External forces can change the energy of a body.
(c) False. Only work done by conservative force over a closed loop is zero.
(d) True. In an inelastic collision, the final velocity reduces, resulting in loss of the initial kinetic energy.
New answer posted
8 months agoContributor-Level 10
6.6 (a) When a conservative force does positive work on a body, the body gets displaced in the direction of the force, it moves towards the centre of the force, thus resulting in decrease of potential energy.
(b) When the work done by a body against friction, it reduces its velocity. Hence kinetic energy decreases.
(c) The momentum cannot be changed by the internal forces on the system; the change of momentum is proportional to the external force.
(d) The total linear momentum does not change in an elastic collision.
New answer posted
8 months agoContributor-Level 10
6.5 (a) As per the law of conservation of energy,
Total energy = potential energy + kinetic energy
= mgh + mv2
When the casing burns, mass reduces, resulting in drop of energy. Hence the energy for burning of casing is drawn from the rocket.
(b) The force due to gravity is a conservative force. The work done on a closed path for a conservative force is zero. Hence, for every complete orbit of the comet, the work done by the gravitational force is zero.
(c) The potential energy of the satellite revolving the Earth decreases as it approaches the Earth and since the system's total energy should remain constant, the k
New answer posted
8 months agoContributor-Level 10
6.4 Given, particle energy, E = 1 J,
Force constant, k = 0.5 N/m
Kinetic energy, KE = mv2
From the equation, total energy, E = KE +PE, we get
1 = (1/2)mv2 + (1/2) kx2
when it turns back, v becomes 0
1 = (1/2) , x = ± 2
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
6.3 We know the total energy E is given by E = Kinetic energy (KE) + Potential energy (PE)
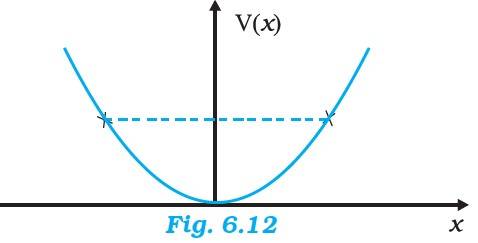
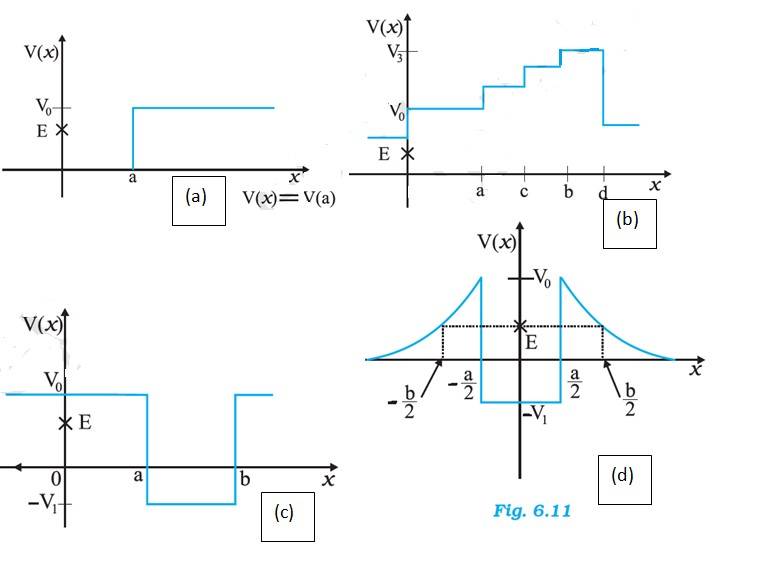
(a) In figure (a), we have at x=0, the potential energy is zero. So KE is positive. At x>a, the potential energy has a value greater than E, so the KE becomes 0. Thus the particle will not exist in the region x>a. Minimum total energy is zero.
(b) For the entire x-axis, PE >E, the KE of the object would be negative. Thus the particle will not exist in this region.
(c) In x=0 to x=a and x>b, PE is greater than E, so he KE has to be negative. The object cannot exist in this region.
(d) For x=-b/2 to x =-a/2 and x=a/2 to x=b/2, KE
New answer posted
8 months agoContributor-Level 10
6.2 Given, mass of the body, m = 2 kg
Horizontal force applied, F = 7 N
Coefficient of friction, = 0.1
Acceleration, a = F/m = 7/2 = 3.5 m/s2
Frictional force, f = 0.1 1.96 N
Retardation produced by the frictional force, = -f/m = -1.96 /2 = 0.98 m/s2
The net acceleration by which the body moves forward
= a - = 3.5 – 0.98 = 2.52 m/s2
Distance moved by the body in 10 s is given by
s = ut + (1/2) = 0 = 126 m
(a) Work done in 10 s is given by
W = Force
(b) Work done by friction in 10 s is given by
W = -f - 247J
(c) Work done by the net force
New answer posted
8 months agoContributor-Level 10
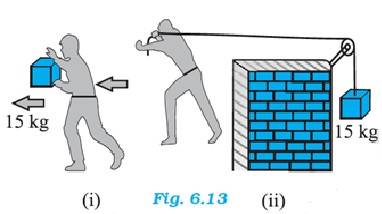
6.1 (a) While the person lifts a bucket out of a well by means of a rope tied to the bucket, the direction of both the force and the displacement are same, hence the work done is positive.
(b) While lifting the bucket, he works against gravity, but the work done by the gravitational force is downward, hence the work done is negative.
(c) The direction of motion of the object is in the opposite direction of the frictional force; hence the work done is negative.
(d) While a body moves on a rough horizontal plane, the frictional forces try to oppose the motion. But since the applied force maintains uniform velocity
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers