Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
13.1 Diameter of the oxygen molecule, d = 3Å
Radius, r = = 1.5 Å = 1.5 cm
Actual volume occupied by 1 mole of oxygen gas at STP = 22.4 lit = 22400
Molecular volume of oxygen gas, V = N,
where N = Avogadro's number = 6.023 molecules/mole
Therefore, V = 6.023 = 8.51
Ratio of the molecular volume to the actual volume of oxygen = = 3.8
New answer posted
8 months agoContributor-Level 10
4.7
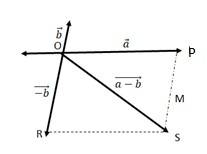
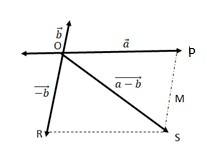
(a) This statement is In order to make a + b+ c + d = 0, it is not necessary to have all four given vectors to be null vectors.
(b) a + b + c +d = 0, ( a + c ) = - (b + d)
Taking the magnitude of both LHS and RHS, | a + c| = | -(b + d)| = | b + d|
So the statement is True.
(c) Given a + b + c + d = 0, a = - (b + c + d)
Taking magnitude of both LHS and RHS, |a| = | -( b + c+ d)| = |b + c +d|
|a| ……….(1)
Equation (1) shows that the magnitude of a can never be greater than the sum of magnitudes of b,c and d. So the statement is True.
(d) In the given expression a + b + c + d = 0, the sum of 3 vectors a, (b+c), d ca
New answer posted
8 months agoContributor-Level 10
(a)
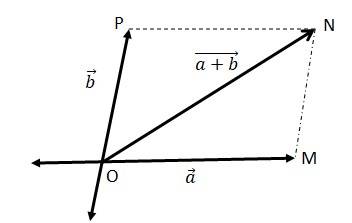
We can write
=
= = ………(ii)
=
In a triangle, each side is smaller than the sum of the other two sides. Therefore in ΔOMN, we have ON < (OM + MN)
< + ….(iv)
If the two vectors and act along a straight line in the same direction, then we can write = +
Combining equations (iv) and (v), we get
+
(b)
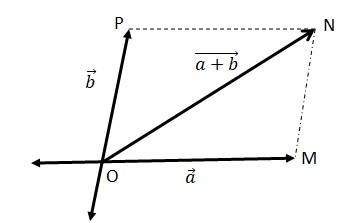
Let two vectors and be represented by the adjacent sides of a parallelogram OMNP.
We can write
=
= = ………(ii)
New answer posted
8 months agoContributor-Level 10
4.5
(a) The magnitude of a vector is a number only, hence always a scalar – TRUE
(b) Each component of a vector is also a vector – FALSE
(c) The total path length is a scalar quantity, whereas displacement is a vector quantity. – FALSE
(d) The total path length is always greater than or equal to the magnitude of displacement of a particle – TRUE
(e) Three vectors which do not lie in a plane can never add up to give a null vector – TRUE
New answer posted
8 months agoContributor-Level 10
4.4
(a) The addition of two scalar quantities is meaningful – if they both represent the same physical quantity.
(b) The addition of a vector quantity with a scalar quantity is not meaningful.
(c) Multiplying a scalar with a vector is meaningful.
(d) The multiplication of two scalar quantities is meaningful.
(e) The addition of two vectors is meaningful.
(f) Adding a component of a vector to the same vector is meaningful.
New answer posted
8 months agoContributor-Level 10
4.3 Impulse is the only vector quantity from the above list. Impulse is the product of force and time. Force is a vector quantity and time is a scalar quantity. The product of 1 vector and 1 scalar is always a vector quantity.
New answer posted
8 months agoContributor-Level 10
4.2 Work & Current are scalar quantities. Work is the product of Force and displacement. Since the product of two vectors quantities are always scalar. Current is independent of direction, it is only magnitude, hence scalar.
New answer posted
8 months agoContributor-Level 10
4.1 A scalar quantity depends only on the magnitude – volume, mass, speeds, density, number of moles, angular frequency are all scalar quantities.
A vector quantity depends on magnitude and direction – velocity, acceleration, displacement and angular velocity are all vector quantities.
New answer posted
8 months agoContributor-Level 10
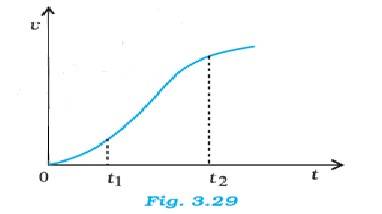
3.28 The graph has a non-uniform slope between the intervals t1 and t2 – the graph is not a straight line. The equations (a), (b), and (e) do not describe the motion of the particle. Only the relations (c), (d) and (f) are correct.
New answer posted
8 months agoContributor-Level 10
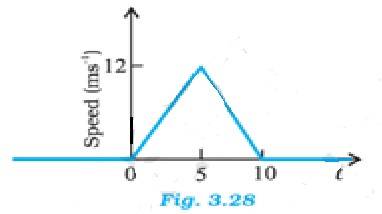
3.27
(a) Distance travelled by the particle between t = 0 s and t = 10 s is the area of the triangle = (1/2) x base x height = (1/2) x 10 x 12 = 60 m
The average speed of the particle is 60/10 m/s = 6 m/s
(b) Distance travelled by the particle between t = 2 s and t = 6 s
Let S1 be the distance travelled by the particle in time 2 to 5 s and S2 be the distance travelled between 5 to 6s.
For the motion from 0 to 5 sec, u = 0, t = 5, v = 12 m/s
From the equation v = u + at we get a = (v-u)/t = 12/5 = 2.4 m/s2
Distance covered from 2 to 5 sec, S1 = distance covered in 5 s – distance covered in 2 s
From the equation s = ut + at2 we
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers