Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
Both lithium and magnesium are harder and lighter than other elements in the respective groups.
Lithium and magnesium react slowly with water. Their oxides and hydroxides are much less soluble and their hydroxides decompose on heating. Both form a nitride, Li3N and Mg3N2, by direct combination with nitrogen.
The oxides, Li2O and MgO do not combine with excess oxygen to give any superoxide. (iv) The carbonates of lithium and magnesium decompose easily on heating to form the oxides and CO2. Solid hydrogen carbonates are not formed by lithium and magnesium.
Both LiCl and MgCl2are soluble in ethanol.
Both LiCl and MgCl2are deliquescent and cry
New answer posted
8 months agoContributor-Level 10
(i) Ionization enthalpy. Because of high nuclear charge the ionization enthalpy of alkaline earth metals are higher than those of the corresponding alkali metals.
(ii) Basicity of oxides. Basicity of oxides of alkali metals are higher than that of alkaline earth metals.
(iii) Solubility of hydroxides of alkali metals is higher than that of alkaline earth metals. Alkali metals due to lower ionization enthalpy are more electropositive than the corresponding group 2 elements.
New answer posted
8 months agoContributor-Level 10
It is because ionization enthalpy? Hi of potassium = 419 kJ mol-1. Ionization enthalpy of sodium = 496 KJ mol-1. Since Ionization enthalpy of potassium is less than that of sodium, potassium is more reactive than sodium.
New answer posted
8 months agoContributor-Level 10
Let x be the oxidation state of Na in Na2O2
Then, 2x + 2 (-1) = 0
=>2x – 2 = 0
=> x = +1.
New answer posted
8 months agoContributor-Level 10
All the alkali metals have one valence electron, ns1 outside the noble gas core. The loosely held s-electron in the outermost valence shell of these elements makes them the mostelectropositive metals, i.e. they readily lose electron to give monovalent M+ ions. Hence, they are never found in free state in nature.
New answer posted
8 months agoContributor-Level 10
The general characteristics and gradation in properties of alkaline earth metals are:
- Atomic size goes on increasing down the group.
- Ionisation energy goes on decreasing down the group.
- They are harder than alkali metals.
- They are less electropositive than alkali metals.
Electropositive character increases on going down the group.
New answer posted
8 months agoContributor-Level 10
- Physical appearance: All the alkali metals are silvery white, soft and light metals.
- Density: Because of the large size, these elements have low density which increases down the group except for potassium which is lighter than sodium (most likely due to an unexpected increase in the atomic size.).
- The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them.
- Atomic volume:The atomic volume, atomic and ionic radii rise as the group number reduces from Li to Cs.
- Melting and boiling points:The weak crystal lattice bonding causes low melting and boili
New answer posted
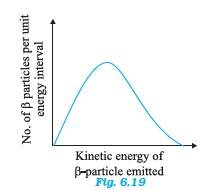
8 months agoContributor-Level 10
6.30 The decay process of free neutron at rest is given as:
n
From Einstein's mass-energy relation, we have the energy of electron as? where,
? m = Mass defect = Mass of neutron – ( Mass of proton + mass of electron)
c = speed of light
? m and c are constant. Hence, the given two-body decay is unable to explain the continuous energy distribution in the -decay of a neutron or a nucleus. The presence of neutrino von the LHS of the decay correctly explain the continuous energy distribution.
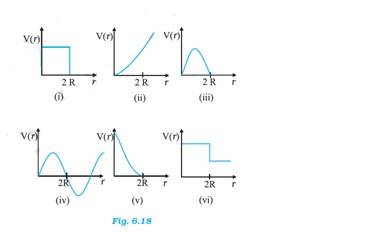
New answer posted
8 months agoContributor-Level 10
6.29 The potential energy of two masses in a system is inversely proportional to the distance between them. The potential energy of the system of two balls will decrease as they get closer to each other. When the balls touch each other, the potential energy becomes zero, I.e. at r = 2R. The potential energy curve in (i), (ii), (iii), (iv) and (vi) do not satisfy these conditions. So there is no elastic collision.
New answer posted
8 months agoContributor-Level 10
6.28 Mass, m = 200 kg
Speed, v = 36 km/h = 10 m/s
Mass of the boy, M = 20 kg
Initial momentum = (M + m)v = (20 + 200) x 10 kg-m/s = 2200 kg-m/s
If v' is the final velocity of the trolley, then
The final momentum = (M+m) x v' – M x 4= 220v'-80
According to the law of conservation of energy,
Initial momentum = final momentum
2200 = 220v'-80
V' = 10.36 m/s
Time required by the boy to travel 10m = 10/4 = 2.5 s
Distance travel by trolley in 2.5 s = 10.36 x 2.5 m = 25.9 m
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers