Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
1.20 ZnS shows Frenkel Defect.
AgBr shows Frenkel Defect and Schottky Defect. Frenkel Defect: It is a kind of defect in crystalline solids in which atoms are displaced from their lattice position
to interstitial site creating vacancy at the lattice point. It usually occurs in ionic solid with large difference in size
of ions.
Schottky Defect: This defect occurs when oppositely charged ions leave their lattice site creating vacancies in
such a way that electrical neutrality of crystal is maintained. It is generally seen in highly ionic compounds where
difference in size of cation and anion is small.
New answer posted
8 months agoContributor-Level 10
1.19 When a solid is heated, vacancy defect can arise. A solid crystal is said to have vacancy defect when some of the lattice sites are vacant. Vacancydefect leads to a decrease in the density of the solid.
New answer posted
8 months agoContributor-Level 10
1.18 Molar mass of the element = 2.7*10-2 kg mol-1
Edge length, a = 405 pm
Density, d = 2.7*103 kg m-3
Using the formula, d=? *? / ? 3NA
Putting the values given at their appropriate place, we get
(2.7 X 103 )X (405 X 10-12)3 X 6.022 X 10 / 2.7*10-2 = 3.99 which is approximately equal to 4
Therefore, it is an fcc unit cell
New answer posted
8 months agoContributor-Level 10
1.17 Hexagonal close-packed lattice has the highest packing efficiency of 74%. The packing efficiencies of simple cubic and body-centered cubic lattices are 52.4% and 68% respectively
New answer posted
8 months agoContributor-Level 10
1.16 The atoms of element M occupy 1/3rd of the tetrahedral voids.
Therefore, the number of atoms of M is equal to 2 1/3 = 2/3rd of the number of atoms of N. Therefore, ratio of the number of atoms of M to that of N is M : N = (2/3):1 = 2:3 Thus, the formula of the compound is M2N3.
ccp= fcc = 6 * 1/2 + 8 X 1/8 = 4 = N atoms
Tetrahedral void= 8 (in fcc)
Number of M atoms = 8/3
So empirical formula = M 8/3 N2 = M2N3
New answer posted
8 months agoContributor-Level 10
1.15 Number of atoms in close packaging = 0.5 mol
1 atom has 6.022*1023 particles
So Number of particles in close-packed = 0.5 * 6.022 * 1023 = 3.011*1023
Number of tetrahedral voids = 2 * number of atoms in close packaging
Number of tetrahedral voids = 2 * 3.011 * 1023= 6.022*1023
Number of octahedral voids = number of atoms in close packaging
So the number of octahedral voids = 3.011 * 1023
Total number of voids = Tetrahedral void + octahedral void
=6.022 * 1023 + 3.011 * 1023= 9.03*1023
New answer posted
8 months agoContributor-Level 10
1.14 In square close-packed layer, a molecule is in contact with four of its neighbours. Therefore, the two-dimensional coordination number of a molecule in square close-packed layer is 4.
New answer posted
8 months agoContributor-Level 10
1.13 (i) An atom located at the corner of a cubic unit cell is shared by eight adjacent unit cells. Therefore, 1/8th portion of the atom is shared by one unit cell.
(ii) An atom located at the body centre of a cubic unit cell is not shared by its neighbouring unit cell. Therefore, the atom belongs only to the unit cell in which it is present i.e., its contribution to the unit cell is 1
New answer posted
8 months agoContributor-Level 10
1.12

(ii) Face-centered unit cell: In a face-centered unit cell, the constituent particles are present at the corners
and one at the centre of each face.
End-centered unit cell: An end-centered unit cell contains particles at the corners and one at the centre of
any two opposite faces.
New answer posted
8 months agoContributor-Level 10
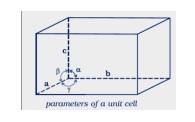
1.11 The six parameters that characterize a unit cell are as follows.
(i) Its dimensions along the three edges, a, b, and c these edges may or may not be equal.
(ii) Angles between the edges these are the angles (between edges b and c), (between edges a and c), and (between edges a and b)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers
