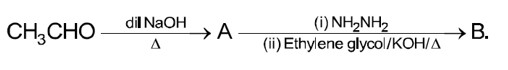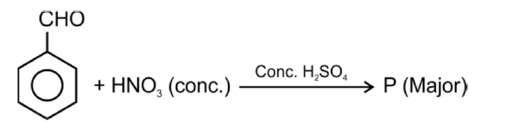Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
2 months agoContributor-Level 10
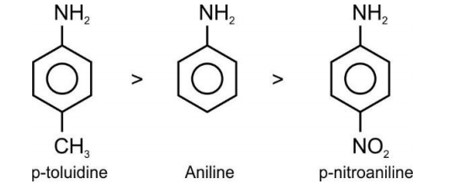
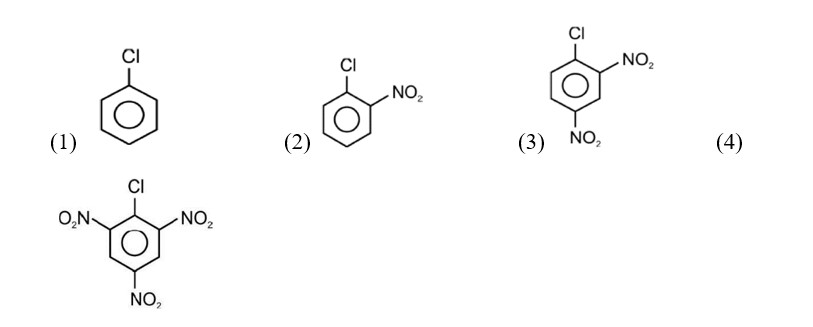
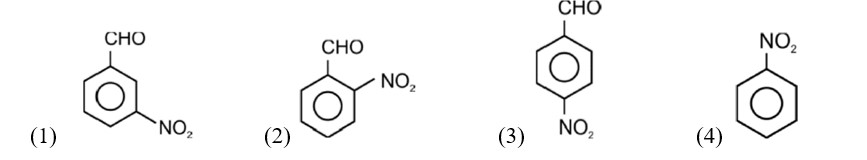
Electron releasing group present at para position increases basic strength.
Therefore, Correct order of basic strength is
New answer posted
2 months agoContributor-Level 10
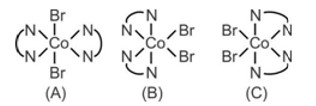
There are three isomers for the complex species [Co (en)2Br2]+
New answer posted
2 months agoContributor-Level 10
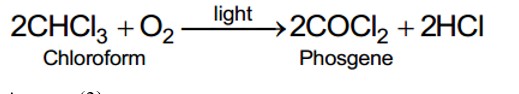
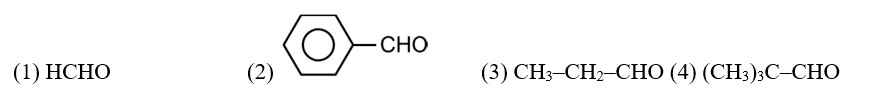
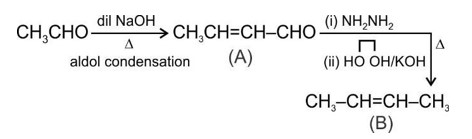
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
New answer posted
2 months agoContributor-Level 10
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers