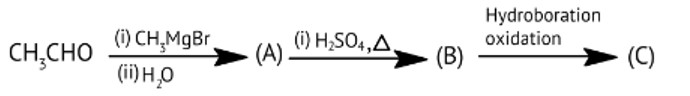Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Solution: (b, d)
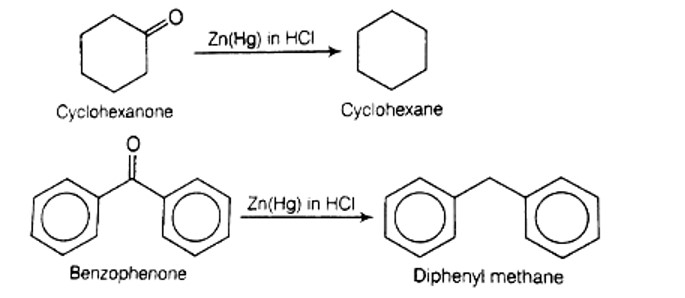
Clemmensen reduction is used to convert cyclohexanone into cyclohexane and benzophenone into diphenyl methane .
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and C
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: B and D
Consider the following product CH3 -CHO C and O are broken into positive and negative bonds. C should have one condition: hydrogen must be available; if it isn't, aldol condensation will not occur. When two products are mixed with the availability of - hydrogen, the positive and negative bonds between carbon and hydrogen are exchanged, and water (H20) is eliminated, resulting in aldol condensation.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: A
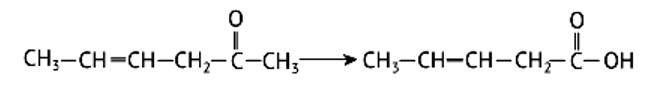
The Clemmensen Reduction method is used to reduce a carbonyl group to CH2. Amalgams are a type of reducing agent that has been around for a long time. In the Clemmensen reduction, hydrogen chloride acts as an acid, protonating the ketone and transferring the electrons from the zinc amalgam to the carbon atoms
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
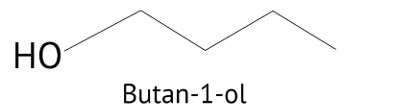
When a primary alcohol, such as Butan-1-ol, reacts with a powerful oxidising agent, such as KMnO4, it produces carboxylic acid, however when a secondary alcohol, such as Butan-2-ol, is oxidised under the pressure of KMnO4, it produces ketone. As a result, option B is right.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: C
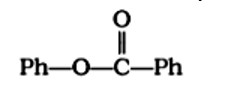
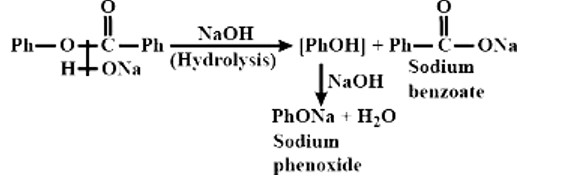
I2 and NaOH It is an iodoform reaction involving methyl ketones, the solution is the correct reagent. Because the reactants and product have double bonds, double bonds do not work throughout the reaction. There are also ketone and carboxylic groups present. The ketonic group is oxidised under the carboxylic group during the process.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
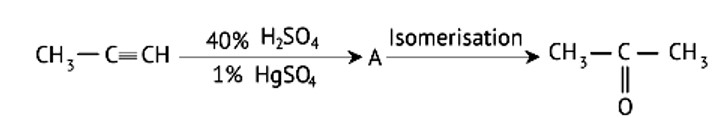
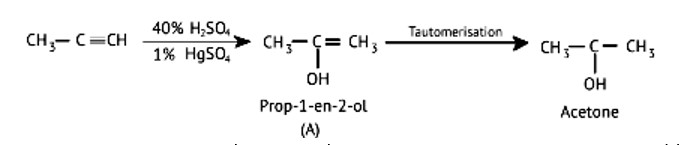
The position of a functional group in the same carbon chain differs in positional isomerism. The functional group in the chemical or the unsaturated double or triple bond in the compound can help us figure it out.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option: D
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct option: B
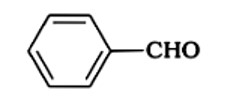
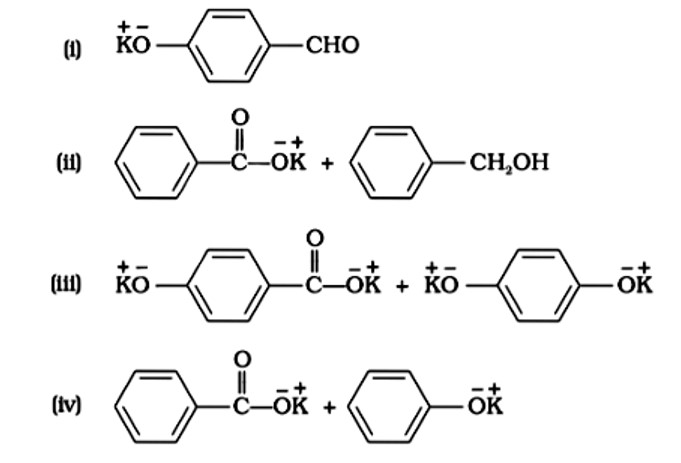
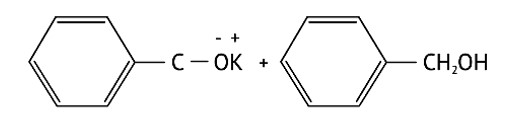
In KOH adds to the compound
Due to the production of a stable conjugate base after hydronium is removed from phenol, it is more stable than alcohol.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Correct options: D
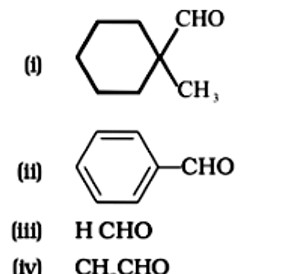
Aldehydes without a - hydrogen atom are the subject of Cannizaro's reaction.
Option D, CH3CHO has 3 alpha hydrogen atoms and it does not give Cannizaro's reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers