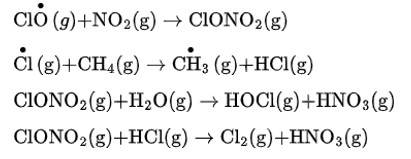Environmental chemistry
Get insights from 152 questions on Environmental chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Environmental chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Option (iii)
i.e., Both A and R are not correct is the answer since in cold places, sunlight required to grow plants is less. Due to which plants are kept in a glass house so that sunlight enters in the greenhouse to heat up the soil and plants. The warm soil and plants emit infrared radiations which are partially absorbed and partially reflected by the glass.
New answer posted
9 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Excessive amount of sulphate (i.e., greater than 500 ppm) in drinking water causes laxative effect, otherwise at moderate levels it is harmless.
New answer posted
9 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Scientists working in the Antarctica region reported that depletion of the ozone layer is commonly termed as an ozone hole over the South Pole in the Antarctic region. It was observed that a unique set of conditions were responsible for the ozone hole. In summer, nitrogen dioxide and methane react with chlorine monoxide and chlorine atoms forming chlorine sinks, preventing much ozone depletion, whereas in winter, special types of clouds called polar stratospheric clouds are formed over Antarctica. These polar stratospheric clouds provide a surface on which chlor
New answer posted
9 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Ozone is found to be thermodynamically unstable and undergoes decomposition into molecular oxygen and a dynamic equilibrium exists between the production and decomposition of ozone. The reactions involved can be shown as-
O2 (g) ![]() O (g) + O (g)
O (g) + O (g)
O (g) + O2 (g) +M![]() O3 (g) + M
O3 (g) + M
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Ozone in the stratosphere is formed when UV radiation falls on the dioxygen (O2 ) molecules. The UV radiations split apart molecular oxygen into free oxygen (O) atoms, these oxygen atoms combine with molecular oxygen to form ozone. The reactions involved can be given as-
O2 (g) ![]() O (g) + O (g)
O (g) + O (g)
O (g) + O2 (g) +M![]() O3 (g) + M
O3 (g) + M
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
NO2 absorbs energy from sunlight and breaks up into nitric oxide and free oxygen atom and this can be shown as-
NO2 (g) NO (g)+O (g)
Here the oxygen atom produced is very reactive and combines with oxygen molecule in the air to give rise to ozone.
O (g) +O2 (g) + M → O3 (g) + M
Here 'M' is an inert gas like Nitrogen gas and ozone gets produced during the formation of smog.
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The uncatalyzed oxidation of SO2 is a slow process. However, the presence of particulate matter in the polluted air catalyses the oxidation of SO2 to SO3. The reaction for the conversion can be given as-
2SO2 (g)+O2 (g)→2SO3 (g)
The reaction can be promoted by O3 or by H2O2.
SO2 (g)+O3 (g)→2SO3 (g)+O2 (g)
SO2 (g)+H2O2 (l)→H2SO4 (aq)
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The symptoms which were observed in the village indicate that oxides of sulphur and nitrogen are released from the chimneys of the factory. These gases are obtained as the product of combustion of fossil fuels such as coal, gasoline etc. In an automobile engine, at high temperature, when fossil fuel is burnt, dinitrogen and dioxygen get combined to give significant quantities of nitric oxide and nitrogen dioxide. The chemical reactions can be given as-
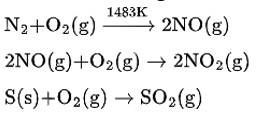
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Due to the excessive growth of algae water contains a lot of phosphate due to the inflow of the fertilizers from the surroundings. The decomposition of algae leads to the bad smell and taste and even makes it unfit for drinking, bathing, washing, swimming, boating etc. and concentration of dissolved oxygen also decreases which may be harmful for aquatic life.
New answer posted
9 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
The amount of BOD of water is the amount of organic material present in water in terms of how much oxygen will be required to break it down biologically. Clean water has a BOD value less than 5 ppm. While highly polluted water has a BOD value of 17 ppm or more.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers