Environmental chemistry
Get insights from 152 questions on Environmental chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Environmental chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Hydrogen peroxide reduces iodine to iodide ion is basic medium as;
New answer posted
5 months agoContributor-Level 9
B. O. D. value < 5 ppm for clean water and B.O.D value of polluted water
New answer posted
5 months agoContributor-Level 10
In stratosphere CFC get broker down by powerful UV/radiation releasing Cl·
New answer posted
5 months agoContributor-Level 10
Assertion : Clean water has BOD less than 5ppm. Highly polluted water has BOD greater than or equal to 17 ppm.
Reason : BOD is measure of oxygen required to oxidize only bio-degradable organic matter.
Thus Assertion (A) is correct & Reason (R) is wrong.
New answer posted
5 months agoContributor-Level 10
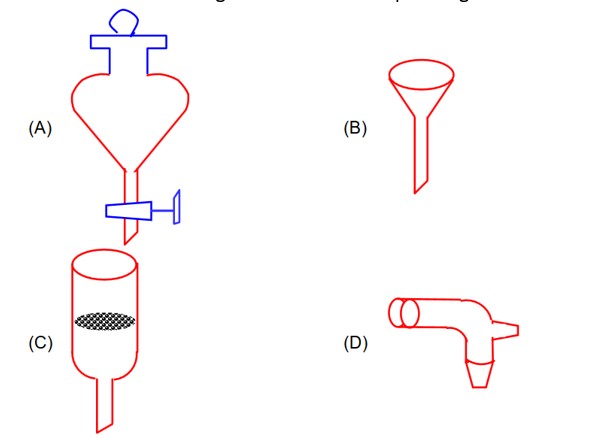
Funnel of option A is useful for the separation of liquid – liquid mixture of immiscible form.
New answer posted
5 months agoContributor-Level 10
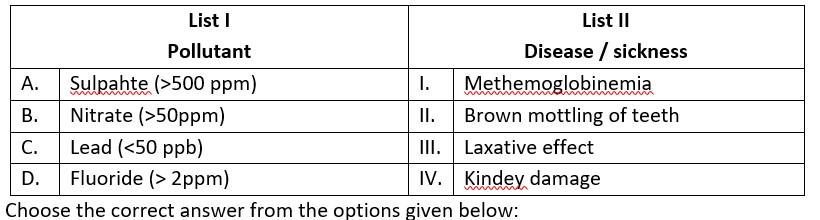
(A) Sulphate (>500 ppm) ® causes laxative effect that leads to dehydration.
(B) Nitrate (> 50 ppm) ® causes methemoglobinemia, skin appears blue.
(C) Lead (>50 ppb) ® It damage kidney and RBC.
(D) Fluoride (>2ppm) ® It causes brown mottling of teeth.
New answer posted
5 months agoContributor-Level 10
(a) Polluted water has low value of dissolved oxygen, but high value of B.O.D., because chemical and organic matter uses dissolved oxygen to decompose.
(b) Eutrophication is result of excessive growth of weed in water bodies, which consumes dissolved oxygen, thus decreases oxygen.
New answer posted
5 months agoContributor-Level 10
In clark's method Ca (OH)2 is used for softening hard water.
Thus CaCO3 and Mg (OH)2 are formed in reaction.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers