NCERT Class 12 Chemistry
Get insights from 19 questions on NCERT Class 12 Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about NCERT Class 12 Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoBeginner-Level 5
The nature of bonding in Ni (CO)? includes synergic bonding. The synergic bonding in metal carbonyls like nickel tetracarbonyl is due to two-way interaction of metal and carbonyl ligand electron density donation.
The synergic bonding is best explained thorugh Molecular Orbital Theory (MOT), which explains the donation of electron density from the filled d-orbitals of nickel (Ni) into the antibonding? * orbitals of the carbon monoxide (CO) ligand.
The synergic bonding can be described as a combination of? -bond formation due to donation of lone pair to metal center, and? back-bond formation due to donation of lone pair to CO ligand.
New answer posted
4 months agoContributor-Level 8
Isoenzymes are the different enzymes that catalyse the same biochemical reaction. However, they differ in amino acid composition and kinetic properties. Examples of isoenzymes are hexokinase and lactate dehydrogenase.
New answer posted
4 months agoContributor-Level 8
There are six types of enzymes. Here we have provided all types of enzymes.
1. Ligases
2. Transferases
3. Hydrolases
4. Oxidoreductases
5. Isomerases
6. Lyases
New answer posted
4 months agoContributor-Level 8
Five enzymes in human body are mentioned below:
1. Amylase
2. Lipase
3. Trypsin
4. Lactase
5. Pepsin
New answer posted
5 months agoBeginner-Level 5
We cover Physics, Chemistry, and Maths in our Class 12 CBSE Notes. Here you can check the overview of the covered topics in our Class 12 Notes.
Class 12 Physics Notes include Electric Charges & Fields, Current Electricity, Magnetic Effects of Current, EM Induction, Optics (Ray & Wave), Dual Nature of Radiation/Matter, Atoms and Nuclei, Semiconductor Electronics, etc.
Class 12 Chemistry Notes includes chapters like Solutions, Electrochemistry, Chemical Kinetics, d? & f? Block Elements, Coordination Compounds, Haloalkanes & Haloarenes, Alcohols, Phenols & Ethers, Aldehydes, Ketones & Carboxylic Acids, Amines, Biomolec
New answer posted
5 months agoBeginner-Level 5
Students should prepare complete syllabus when they have time to prepare. However, you can use the list of highweightage chapters in last minute revision for scoring well.
- The p-Block Elements: This chapter holds a high weightage of 8–10 marks. (in the latest syllabus this is deleted)
Aldehydes, Ketones, and Carboxylic Acids: This chapter contributes around 8–10 marks.
Biomolecules: This chapter accounts for around 8 marks.
Chemical Kinetics: This chapter holds a high weightage of 5-6 marks.
The d- and f-Block Elements: This chapter contributes around 5-6 marks.
Amines: This chapter contributes around 5-6 marks.
New answer posted
5 months agoContributor-Level 10
Be2Cl4 is Lewis acid and Al2Cl6 has complete octet. Be and Al are amphoteric metal therefore dissolve in acid as well as alkaline solution and form beryllate and aluminate ions in excess alkali.
New answer posted
5 months agoContributor-Level 10
are paramagnetic
Weakly attracted by magnetic field
NaCl and H2O are diamagnetic
Weakly repelled by magnetic field.
New answer posted
6 months agoContributor-Level 10
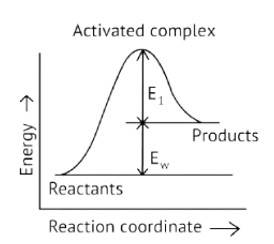
This is a Fill in the blanks Type Question as classified in NCERT Exemplar
Ans: Correct option A
Activation Energy → The amount of energy required to overcome the obstacle and generate a product
The activation energy of a forward reaction can be seen in the [Eaf = E1 + E2] (This is an endothermic reaction.
Therefore, [Eaf > Eab]
The energy of the product is high, while the energy of the reactant is low.
The lower the energy, the more stable and positive the situation becomes.
Δ? = posistive
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers