Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
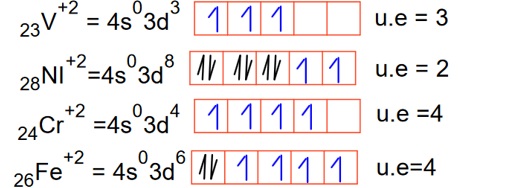
the lowest spin only magnetic moment for Ni+2 because of minimum unpaired electron.
New answer posted
3 months agoContributor-Level 10
For AB3 interhalogen compound, which is T-shaped, only two lone pair electrons are available on central atom.
New answer posted
3 months agoContributor-Level 10
Electronic configuration of
Gd is ; Gd64 = [Xe]4f7 5d1 6s2
Hence
Total number of electrons in 4f sub-shell = 7
Ans. = 7
New answer posted
3 months agoThe ratio of number of water molecules in Mohr's salt and potash alum is_____* 10-1
(Integer answer)
Contributor-Level 10
Molecular formula of Mohr' salt, FeSO4. (NH4)2SO4.6H2O.
Molecular formula of potash alum = K2SO4.Al2 (SO4)3.24H2O. Overall molecular formula of potash alum = KAl (SO4)2. 12H2O. Ratio of water molecules in Mohr' salt and Potash alum
Ans. = 5
New answer posted
3 months agoContributor-Level 9
In electro-refining, impure metal (blister copper) is used as an anode while precious metal like Au, Pt gets deposited as anode mud.
New answer posted
3 months agoContributor-Level 10
Reactivity order of halogens and inter halogens are F2 > ClF > Cl2 and ClF forms HOCl and HF after the reaction with water.
New answer posted
3 months agoContributor-Level 10
Statement-I is true, because metal reduced by decreasing
New answer posted
3 months agoContributor-Level 10
Weight = 1.53 gm
V = 448 ml (STP)
Mole =
Molecular weight of compound A = 50 * 1.53 = 76.5 gram
Molecular weight 76.5 = CnH2n-1 Cl
or 12 n + 2n – 1 = 41
Molecular formula = C3H5Cl
Number of C- atoms = 3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers