Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Oxygen is more electronegative than sulphur, the bond angle of H2O is greater, and the bod pair electron of an OH bond will be closer to oxygen, causing bond-pair repulsion between bond pairs of two OH bonds
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Chlorine has vacant d-orbitals, which become excited upon bonding when electrons from the 3p-orbital are promoted to the 3d-orbital, giving it a covalency of three.
Due to the lack of unoccupied d-orbitals in the second energy shell, fluorine cannot expand its octet. As a result, it can only have one covalency.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
NO2 exists as a monomer with one unpaired electron in the gaseous state, but it dimerises to N2O4 in the solid state, leaving no unpaired electron, making the solid form diamagnetic.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
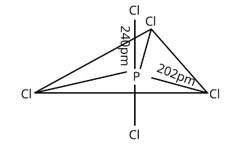
Three P—Cl bonds are arranged in one plane at a 1200 angle. These bonds are known as equatorial bonds because they are formed between two points. The remaining two P—Cl bonds, one above the equatorial plane and the other below it, form 90° angle with the plane. Axial bonds are the name for these types of bonds. Because axial bond pairs are subjected to greater repulsive interaction than equatorial bond pairs, axial bonds are slightly longer and hence slightly weaker than equatorial bonds, making the PCl5 molecule more reactive.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
PH3 is insoluble in water and cannot create hydrogen bonds with it, it forms bubbles, whereas NH3 dissolves because it is soluble in water and can form hydrogen bonds with it.
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
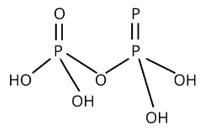
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
4NH3 + 5O2 ![]() 4NO + 6H2O
4NO + 6H2O
New answer posted
5 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
SO3 generates a dense fog of sulphuric acid that does not condense quickly, it is not absorbed directly in water to form H2SO4.
New answer posted
5 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
A = NH4NO2
B = N2
C = NH3
D = HNO3
(i) NH4NO2→N2 + 2H2O
(ii) N2 + 3H2→2NH3
(iii) 4NH3 + 5O2→4NO + 6H2O
4NO + O2→4NO2
3NO2 + H2O→2HNO3 + NO
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
