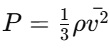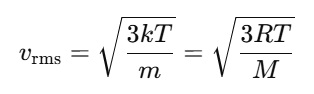Physics Spl
Get insights from 6.8k questions on Physics Spl, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Spl
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
10 months agoNew answer posted
10 months agoContributor-Level 10
The main difference between the real and ideal gases is with respect to the kinetic theory. The ideal gas follows assumptions of the kinetic theory but the real gases do not follow it, especially when the temperature is low and there is high pressure.
The following points are applicable in the case of real gases:
- Real gas molecules do have finite volume.
- In such cases, intermolecular forces such as Van there Waals forces become significant.
New answer posted
10 months agoContributor-Level 10
According to the kinetic theory of gases class 11, the Maxwell-Boltzmann distribution law describes the gas molecules' distribution of speeds (or velocities). All molecules of gas do not move at the same speed, some move fast, some are slow and most move at an average speed. This law provides a mathematical function that helps in finding at a given temperature, how many molecules have a particular speed. The Maxwell-Boltzmann distribution law helps in defining three important types of speeds:
- Average speed: Arithmetic mean of all speeds.
- Most probable speed: Speed at which the maximum number of molecules travel.
- Root mean square (RMS
New answer posted
10 months agoContributor-Level 10
According to the NCERT Solutions for Class 11 Physics Chapter 12 Kinetic Theory, the pressure is exerted due to gas molecules' collisions with the walls of the container. Each gas molecule has a certain mass and velocity. According to Newton's law, when the molecules collide with a wall, it changes momentum and this change in momentum exerts a force on the container wall. By considering the collisions from all molecules over a period in a unit area, the average force per unit area is called pressure. Mathematically, pressure P is:
p is the gas density.
New answer posted
10 months agoContributor-Level 10
The kinetic theory talks about the molecular explanation for the classical gas laws:
Boyle's Law (P? 1/V at constant T): Gas molecules will exert more pressure when the volume of the container reduces as it has less space to move and hence it collides with the walls of the container. However, when the temperature is constant, the speed of the molecule will not change.
Charles's Law (V? T at constant P): With the increase in temperature, the speed and average kinetic energy of the molecules increase. The gas expands to maintain constant pressure and increases the volume.
The kinetic theory links the physical behaviour of gases with the mo
New answer posted
10 months agoContributor-Level 10
In a sample, Root mean square (RMS) speed refers to the average of the squares of the speeds of all gas molecules. It helps in measuring the gas particles' speed and takes into account the kinetic energy. Mathematically, it is expressed as:
Here, T is the temperature in Kelvin, k is the Boltzmann constant, and m is the mass of one molecule. M is the molar mass and R is the universal gas constant. The RMS speed is calculated by kinetic theory using the relationship between molecular motion, kinetic energy and pressure. RMS speed shows with which speed the molecules are moving in a gas sample and increases with temperature.
New answer posted
10 months agoContributor-Level 10
The official answer key for KCET 2025 Physics (Paper A1) is expected to be released by the Karnataka Examination Authority (KEA) around April 30, 2025. Candidates can download the answer key from the official KEA website at cetonline.karnataka.gov.in. To access the answer key, visit the website, navigate to the 'Answer Key' section, and select the appropriate subject and version code. This will allow you to download the Physics answer key in PDF format?
Please note that the provisional answer key will be released first, followed by the final answer key after considering any objections raised by candidates. The final answer key is typica
New answer posted
10 months agoContributor-Level 10
The kinetic theory is based on many key assumptions, such as the following:
- The individual gas molecules volume is almost non-existing when compared to the total volume of the gas.
- A gas consists of many small, identical, hard spherical particles.
- The collisions between the gas particles and the container walls are perfectly elastic.
- Except during the collisions, there are no intermolecular forces between the atoms.
- The time spent in collisions is almost nil when compared to the time between collisions.
- The gas molecules are in random and constant motion.
- The average kinetic energy of the molecules is directly proportional to the absolute tem
New answer posted
10 months agoContributor-Level 10
Escape velocity is the least velocity an object needs without any further propulsion to escape the Earth's gravitational field. The escape velocity for the earth is nearly 11.2 km/s. It is derived by using the conservation of energy principle that says for an object to move to an infinite distance, its kinetic energy should be equal to the gravitational potential energy.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers