Physics
Get insights from 5.6k questions on Physics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
U = mV (r) = -Cm/r
F = -dU/dr = -Cm/r² ⇒ The force which provides required centripetal force
⇒ mv²/r = Cm/r² ⇒ r = C/v²
New answer posted
4 months agoContributor-Level 10
I = (V/R) (1 - e^ (-t/τ) where τ = L/R
E = (1/2)LI² = (1/2)L (V²/R²) (1 - e^ (-t/τ)² ⇒ Energy stored in inductor
According to Question, we can write
(1/4) * (1/2) (LV²/R²) = (1/2) (LV²/R²) (1 - e^ (-t/τ)²
⇒ 1/4 = (1 - e^ (-t/τ)² ⇒ 1/2 = 1 - e^ (-t/τ) ⇒ e^ (-t/τ) = 1/2
⇒ t/τ = ln (2) ⇒ t = τln (2) = (L/R)ln (2)
New answer posted
4 months agoContributor-Level 10
R = mv / (qB) = √ (2mK) / (qB) ⇒ K = (R²q²B²) / (2m)
⇒ K? /Kα = (R? ²q? ²B² / 2m? ) * (2mα / (Rα²qα²B²) = (mα/m? ) * (R? /Rα)² * (q? /qα)²
⇒ K? /Kα = (4) * (2)² * (1/2)² = 4:1
New answer posted
4 months agoContributor-Level 10
R = (2μ sinθ) / (1.22λ) . (1)
According to de-Broglie's hypothesis, we can write
λ = h / (mv) . (2)
With the help of equations (1) and (2), we can write
R = (2μmv sinθ) / (1.22h) ⇒ R? /R? = m? /m? = 1837 ⇒ R? = 1837R?
- Concept involved: Resolving Power of Microscope
- Topic: Optics
- Difficulty level: Moderate
- Point of Error: Formula
New answer posted
4 months agoContributor-Level 10
- Concept involved: Electric and magnetic field lines
- Topic: Magnetics and Magnetic Material
- Difficulty level: Moderate
- Point of Error: Fact
New answer posted
4 months agoContributor-Level 10
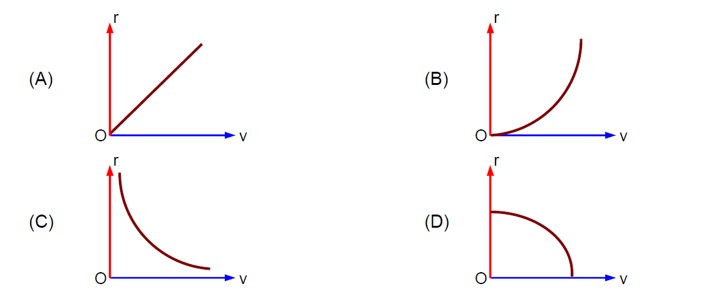
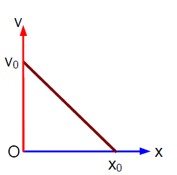
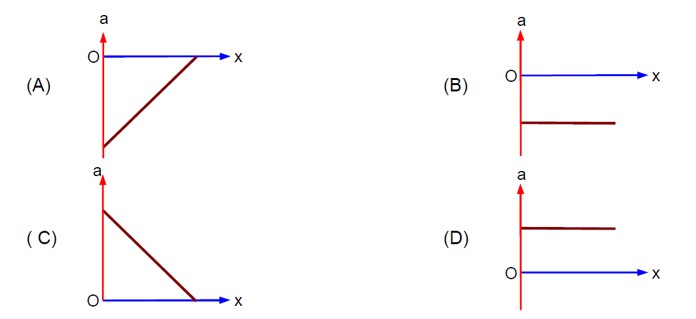
(v/v? ) + (x/x? ) = 1 ⇒ v = - (v? /x? )x + v?
⇒ a = dv/dt = - (v? /x? ) (v) = - (v? /x? ) [- (v? /x? )x + v? ] ⇒ a = (v? ²/x? ²)x - v? ²/x?
- Concept involved: Graph of kinematics
- Topic: Kinematics
- Difficulty level: Moderate
- Note: IIT-Jee-2005
- Point of Error: Writing Equation of straight line and differentiation
New answer posted
4 months agoContributor-Level 10
When white light passes through a cool gas, atoms absorb specific wavelengths. This produces a continuous background with dark lines at those absorbed wavelengths. This is atomic absorption spectra. But when atoms are excited, they release photons at specific wavelengths, producing a dark background with bright lines. That is emission spectra.
New answer posted
4 months agoContributor-Level 10
Every element has a unique set of spectral lines as its electrons occupy specific energy levels. What scientists know for certain is that these unique patterns act like fingerprints. It helps in identifying elements in stars, flames, or unknown samples. For instance, Helium was discovered in the Sun's spectrum before it was found on Earth.
New answer posted
4 months agoContributor-Level 10
We see a discrete emission spectrum when electrons inside excited atoms or ions in gases fall back from higher energy levels to lower ones. Every transition releases a photon of a specific wavelength. Usually the spectrum appears as sharp, bright lines. Dark gaps separate them. These lines are unique to each element.
On the other hand, a continuous emission spectrum is produced when hot solids, liquids, or dense gases emit radiation. This happens because of the collective motion of their atoms and electrons. We don't see sharp lines. Instead the spectrum shows a smooth and unbroken spread of all wavelengths.
New answer posted
4 months agoContributor-Level 9
While the particle moves from mean position to displacement, half of its amplitude, its phase changes by π/6 rad. So,
Time taken, t = (π/6)/ω = T/12 = (2/12)s = (1/6)s
a = 6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers