Physics
Get insights from 5.6k questions on Physics, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- R= 12dis/min per gm and R0= 16dis/min per gm T1/2= 5760yr
R=
By taking log
= log1016/12
= 2.303 (0.6020-4.771)5760/0.6931
= 2391.20yr
New answer posted
7 months agoContributor-Level 10
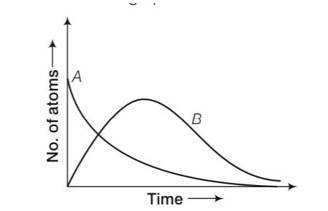
by considering the graph at t=0 NA= No while NB=0 but when time increases the atoms in B also increases and becomes maximum and then drop to zero by radioactive decay law.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation-because more number of protons means more repulsive force which leads to instability.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- during the collision of electron and positron they form gamma radiation and these gamma particle travels in opposite direction to conserve the momentum.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- electron cannot emit radiation while exiting because they absorbs energy in eV which is very low as compared to gamma particle . as they contain energy in MeV
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
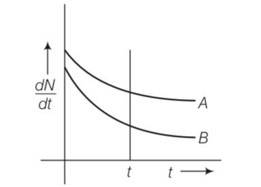
Explanation- at t=0 (dN/dt)A= (dN/dt)B
By drawing a perpendicular line across graph (dN/dt)A> (dN/dt)B so decay is faster in A than B
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
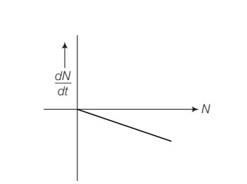
Explanation- dN/dt=
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Explanation- nuclei 2He4 and 1He3 have the same mass number . the element having more number of proton having more repusion so less binding energy. So 2He4 have more number of proton so more repulsion so less binding energy.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- B.E = (Mp+MH-MN)c2
B.E= (118.9058+1.0078252-119.902199)c2
B.E=0.0114362 c2
B.E= (Mp+MH-MN)c2
B.E= (119.902199+1.0078252-120.902822)c2
B.E= 0.0059912c2
(ii) the existence of magic numbers indicates that the shell structure of nucleus is similar to the shell structure of an atom. This also explains peaks in binding energy curve.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
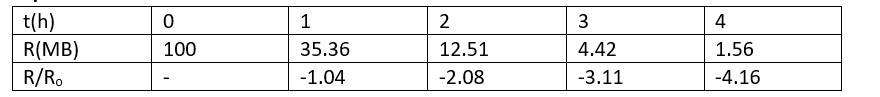
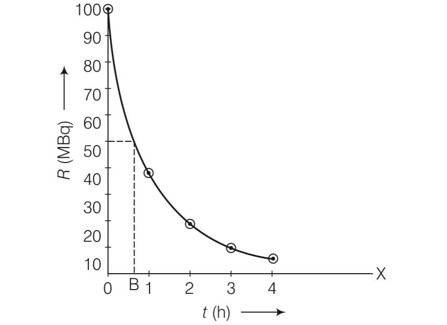
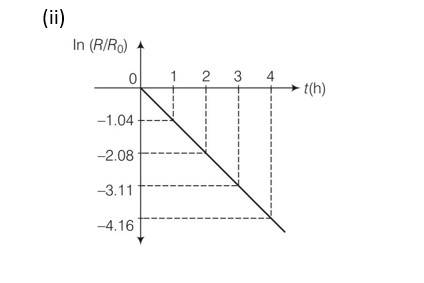
Explanation-
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers