Some Basic Concepts of Chemistry
Get insights from 131 questions on Some Basic Concepts of Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Some Basic Concepts of Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
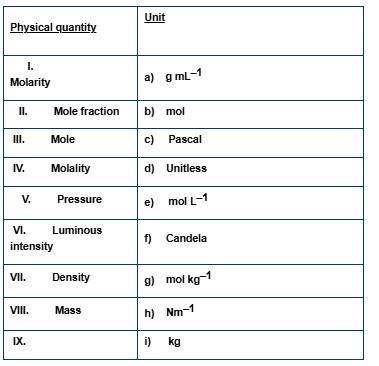
This is a Matching Type Questions as classified in NCERT Exemplar
(i) → (e) (ii) → (d) (iii) → (b) (iv) → (g) (v) → (c), (h) (vi) → (f) (vii) → (a) (viii) → (i)
Explanation:
(i) It is defined as the number of moles of solute dissolved in 1 litre of solution. So, the unit of molarity is mol L-1
(ii) Mole fraction is defined as the number of moles of a constituent divided by the total number of moles. So, it is unitless.
(iii) Mole is the unit to measure the amount of an atom or molecule in SI system. it is symbolized as "mol".
(iv) It is defined as the number of moles of solute dissolved in 1 kg of solvent, so, the unit o
New answer posted
8 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (b); (ii) - (c); (iii) - (a); (iv) - (e); (v) - (d)
(i) 88 g of CO2
The number of moles is given by the following formula,
Moles = - (1)
So, the number of moles of CO2 is calculated by using equation (1) as follows
Moles of CO2 = = 2 mol
Thus, option (i) from column I is matched with (b) from column II
(ii) 1 mol of H2O gives 6.022*1023 molecules. So, 6.022*1023 molecules contain 1 mol of H2O . Thus, option (ii) from column I is matched with (b) from column II
(iii) 5.6 liters of O2 at STP
1 mol of gas occupies 22.4 liters of O2 . For 5.6 lit
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A), (D)
Law of conservation of mass: According to this law, the mass is neither created nor destroyed during a chemical reaction in an isolated system. It can only be transformed from one form to another. Burning of wood is an example of conservation of mass. Law of definite proportion:
According to this law, a compound contains exactly the same elements in the fixed proportion by mass. For example, pure water consists of 11.196 H and 88.9% o by mass.
Law of multiple proportion: According to the law of multiple proportion, when two elements react to form two
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C), (D)
Molality: It is defined as the number of moles of solute divided by the mass of solvent in kg . It is denoted by m . The formula is expressed as
m =
The SI unit of molality is 1 mol/kg
Molarity: It is defined as the number of moles of solute divided by the volume of solution in latice. It is represented by M The formula of molarity is expressed as
M =
Here, n is the number of moles of solute and V is the volume of solution expressed in liters. The SI unit of molarity is mol/ L .
Mole fraction (x ) : The mole fraction of a substance is defined as the n
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option(C), (D)
The number of moles is given by the following formula,
Moles = ---------------(1)
The number of molecules can be calculated as, number of moles
= ----------(2)
(C) The number of moles of N2 is calculated by using equation (1) as follows,
Moles of N2 = = 0.5 mol
The number of molecules can be calculated by using equation (2) as follows,
0.5 mol =
number of molecules = 0.5 6.022 1023
(D) The number of moles of H2 is calculated by using equation (1) as follows,
Moles of H2 = = 0.5 mol
The number of molecules can be calculat
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
The answers are options (i) and (ii)
(A)The number of moles is given by the following formula,
Moles =
So, the number of moles of NaOH is calculated, by using equation (1) as follows
20 g NaOH in 200 mL solution.
Moles of NaOH= mol of NaOH
The molarity (M ) is given by the formula:
M=
On substituting the values in the above equation:
M (NaOH) =
= 2.5 mol L-1
(B) The molarity is given by the formula:
M=
On substituting the values in the above equation:
M (KCL) =
= 2.5 mol L-1
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option(C), (D)
(A) The number of moles is given by the following formula,
Moles = -----------(1)
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 = = 0.5 mol
The number of atoms can be calculated as, number of moles
-----------(2)
On substituting the values in the equation (2)
0.5 mol =
number of atoms = 0.5 6.022 1023
The number of moles of H2 is calculated by using equation (1) as follows,
Moles of H2 =
= 2 mol
On substituting the values in the equation (2):
2 mol =
number of atoms = 2 6.022 1023
(B) The number of moles o
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B), (C)
0.1 mole H2SO4 reacts with 1 mole of NaOH.
0.1 mole of NaOH will react with = mole of H2SO4
Here, NaOH is the limiting reagent.
2 mole of NaOH produces 1 mole of Na2SO4
0.1 mole of NaOH will give mole of Na2SO4
No. of mole =
On substituting the value in the above equation, the mass can be calculated as
0.05 mol =
given mass = 7.10 g
Volume of solution after mixing is 2 L.
So, the molarity of Na2SO4 is
Molarity = = 0.025mol L-1
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A), (D)
One mole of oxygen gas at STP is equal to 6.022 x 1023 molecules of oxygen The number of moles is given by the following formula,
Moles = ----------(1)
The number of moles of O2 is calculated by using equation (1) as follow.
Moles of O2 =
= 0.5 mol
The number of molecules can be calculated as, number of moles
= ----------(2)
On substituting the values in the above equation:
0.5 m
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
Law of multiple proportion: According to the law of multiple proportion, whe form two or more than two chemical compounds, the ratio between different elements combining with a fixed mass of the other is always in the ratio of tin Compounds of carbon and oxygen:
Carbon and oxygen react to form two different compounds CO and CO2. In Carbon react with 16 parts by mass of oxygen.
In CO2 ,12 parts by mass of Carbon react with 32 parts by mass of oxygen. If the mass of Carbon is fixed at 12 parts of mass then the ratio in the masses with the fixed mass of
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers