Structure of Atoms
Get insights from 148 questions on Structure of Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Cu (c) 1s2 2s2 2p6 3s2 3p6 3d10 4s1
(ii) Cu2+ (d) 1s2 2s2 2p6 3s2 3p6 3d9
(iii) Zn2+ (a)1s2 2s2 2p6 3s2 3p6 3d10
(iv) Cr3+ (e) 1s2 2s2 2p6 3s2 3p6 3d3
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i)& (iv)
- Principal quantum number (n): It represents the size and energy of orbitals.
- Azimuthal quantum number (l): It represents the subshell and shape of orbitals.
- Magnetic quantum number (ml): It represents the orientation of the orbitals.
- Spin quantum number (ms): It represents the direction of spin of electrons in the orbitals.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i) & (iii)
Isoelectronic species are those species that possess the same number of electrons. Here both Na+, Mg2+ possess 10 electrons and both Na+, O2– possess 10 electrons.
New answer posted
7 months agoContributor-Level 10
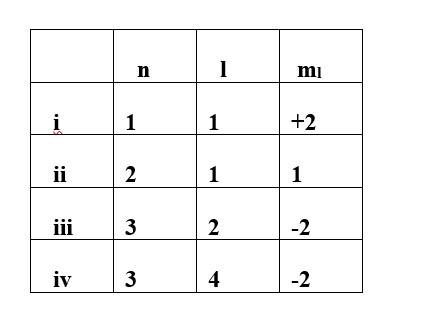
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii) & (iii)
For the given value of n (principal quantum number) the value of l (Azimuthal quantum number) varies from 0 to n-1. However for the given value of l the ml (magnetic quantum number) varies from -1 to +1.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i) & (iv)
In a multielectron atomic system the energy of an electron depends not only on its principal quantum number (shell), but also on its azimuthal quantum number (subshell). Electrons having the same shells and same subshells have the same energy and they are known as degenerate orbitals.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) & (iv)
The isotopes are defined as atoms with identical atomic numbers but different mass numbers are known as isotopes.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii) Alpha particle (He2+)
As the wavelength is inversely proportional to the mass of the particles thus the alpha particles would possess the shortest wavelength among the above given option
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iv) The two electrons present in the 2s orbital have spin quantum numbers MS but of opposite sign.
According to Pauli's Exclusion Principle no two electrons should possess the same set of four quantum numbers. Thus the two electrons present in the 2s orbital have spin quantum numbers MS but of opposite sign.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii)
Fe3+, Mn2+ Fe3+ electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d5 4s2 Mn2+electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d5 4s
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii) 1:3
The chlorine is a mixture of two isotopes with atomic masses 37U and 35U and its atomic mass is 35.5U. The atomic mass depicts that % composition of Cl-35 is much higher than that of Cl-37 and they are present in the ratio 1:3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers