Chemistry NCERT Exemplar Solutions Class 11th Chapter Two Structure of Atom is an extremely useful practice material for Class 12 Science students who are preparing for the CBSE Board exams and other entrance exams. The exemplar goes beyond the NCERT textbook and contains all types of questions around the main topics of the Structure of Atom. The advanced-level questions are based on subatomic particles, atomic models, electronic configuration, and quantum mechanical concepts. The solutions given in the exemplar help students to understand key topics like Heisenberg’s uncertainty principle, de Broglie’s hypothesis, Bohr’s model, orbital shapes, and quantum numbers.
Students can also download the Class 11 Structure of Atom PDF from this page, which has well-structured solutions of the NCERT Exemplar book. It has solutions to all the Multiple-Choice Questions, short-answer type, Matching Type, Assertion and Reason Type, and Long Answer Type Questions. By practicing from this PDF, students will improve their problem-solving skills and will be more confident while solving questions in exams.
Students must also check the NCERT Solutions for the Class 11th Chapter Two: Structure of Atom.
- Download PDF of NCERT Exemplar Class 11 Chemistry Chapter Two Structure of Atom
- Structure of Atom Chemistry Class 11 NCERT Exemplar Short Answer Type Questions
- Class 11 Chemistry Structure of Atom NCERT Exemplar Long Answer Type Questions
- Structure of Atom Class 11 Chemistry Matching Type Questions
- NCERT Exemplar Structure of Atom Class 11 Assertion and Reason Type
- NCERT Exemplar Class 11 Chemistry Structure of Atom Objective Type Questions
- FAQs Related to Chemistry Chapter Two NCERT Exemplar
- Common Mistakes and Tips for NCERT Chemistry Exemplar Chapter Two
- JEE Mains 2022
Download PDF of NCERT Exemplar Class 11 Chemistry Chapter Two Structure of Atom
The PDF offers easy offline access. Once the student downloads the Class 11 Structure of Atom PDF, they can access the solutions even without the need for an internet connection. The PDF is created by the subject matter expert which will strengthen your understanding of this chapter. It offers exam-oriented preparation for school exams and NEET and JEE exams. It supports self-study and offers a quick tool for revision.
Also read:
NCERT Solutions for Class 11 Chemistry
Structure of Atom Chemistry Class 11 NCERT Exemplar Short Answer Type Questions
The SA questions require you to reply concisely. It is designed to test your conceptual clarity. The Short Answer Type Questions from the Structure of Atom focus on the nature of electromagnetic radiation, quantum numbers, and basic atomic models. SA questions are best for applying theoretical knowledge to solve real-world problems and strengthening fundamental concepts.
SA Questions
Ans: The effective nuclear charge (Zeff) is defined as the net positive charge experienced by the outermost electrons in the atom. With the increase of Azimuthal quantum number (l) the Zeff experienced by the electron decreases. Hence the arrangement of subshells in the increasing order of Zeff is : d
Ans: The electronic configuration of oxygen atom is 1s2 2s2 2p4 and its orbital diagram is given by

Commonly asked questions
Nickel atom can lose two electrons to form Ni2+ ion. The atomic number of nickel is 28. From which orbital will nickel lose two electrons.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The electronic configuration of nickel is 1s2 2s2 2p6 3s2 3p6 3d8 4s2 , thus it loses electrons from its 4s orbital as its energy is higher compared to the other orbital.
Which of the following orbitals are degenerate?
3dxy , 4dxy , 3dz2, 3dyz , 4dyz ,4dz2
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: In a multielectron atomic system the energy of an electron depends not only on its principal quantum number (shell), but also on its azimuthal quantum number (subshell). Electrons having the same shells and same subshells have the same energy and they are known as degenerate orbitals.
Thus 3dxy ,3dz2 , 3dyz and 4dxy, 4dyz, 4dz2 are degenerate.
Calculate the total number of angular nodes and radial nodes present in 3p orbital
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The number of radial nodes is given by n-l-1, where n is principal quantum number, l is azimuthal quantum number. The number of angular nodes is given by n-l, where n is principal quantum number, l is azimuthal quantum number. Here n =3 and l =1 Thus, angular nodes = 3-1 = 2 and radial node = 3-1-1 = 1.
The arrangement of orbitals on the basis of energy is based upon their (n+l ) value. Lower the value of (n+l ), lower is the energy. For orbitals having same values of (n+l), the orbital with lower value of n will have lower energy.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Based upon the above information, arrange the following orbitals in the increasing order of energy.
(a) 1s, 2s, 3s, 2p
(b) 4s, 3s, 3p, 4d
(c) 5p, 4d, 5d, 4f, 6s
(d) 5f, 6d, 7s, 7p
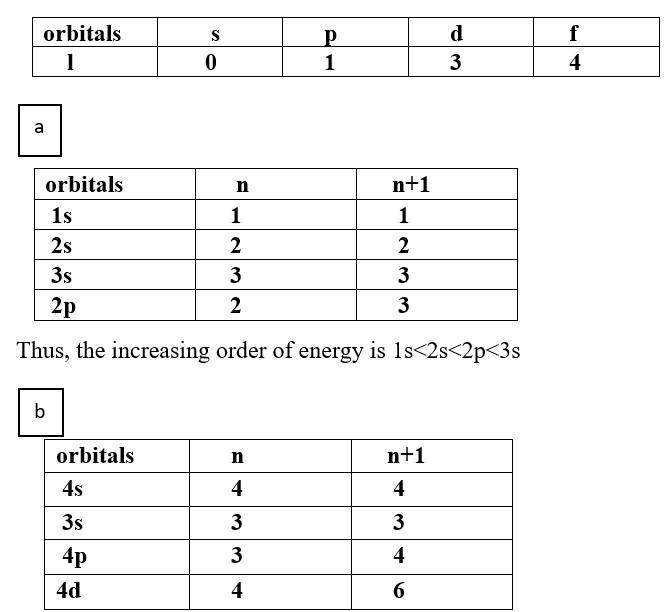
Thus, the increasing order of energy is 3s<3p<4s<4d
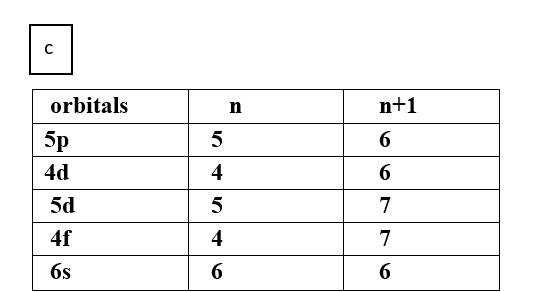
Thus, the increasing order of energy is 4d<5p<6s<4f<5d
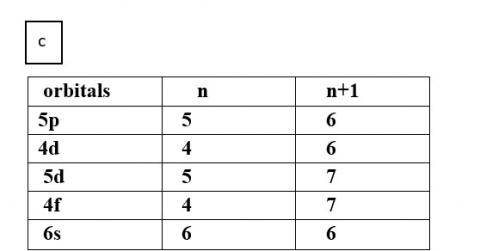
Thus, the increasing order of energy is 7s<5f<6d<7p
(II) Based upon the above information, solve the questions given below :
(a) Which of the following orbitals has the lowest energy?
4d, 4f, 5s, 5p
Ans -
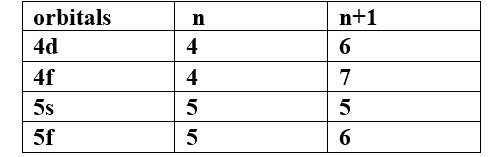
The lowest energy orbital is 5s
(b) Which of the following orbitals has the highest energy?
5p, 5d, 5f, 6s, 6p
Ans-
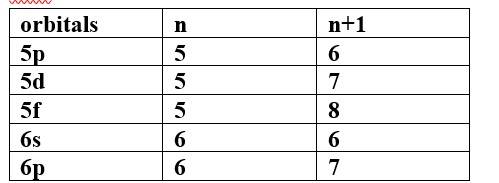
The highest energy orbital is 5f
Which of the following will not show deflection from the path on passing through an electric field?
Proton, cathode rays, electron, neutron
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The charged particles get deflected by the electric field. As among the given option only neutron is the particle that is neutral thus it does not get deflected by the electric field.
An atom having atomic mass number 13 has 7 neutrons. What is the atomic number of the atom?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The mass number (A) is defined as the sum of the number of protons and neutrons present in the nucleus and the atomic number is defined as the number of protons or electrons present in an atom. Thus A=13, the number of neutrons is 7 so the number of protons is 6. Thus, the atomic number is 6.
Wavelengths of different radiations are given below :
λ(A) = 300 nm λ(B)= 300µm λ(C) = 3 nm λ (D) = 30 A0
Arrange these radiations in the increasing order of their energies.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:

We know that energy is inversely proportional to the wavelength

Thus, the increasing order of energy is B
The electronic configuration of valence shell of Cu is 3d104s1 and not 3d94s2 . How is this configuration explained?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: As per the Hund's rule the half filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half filled 4s is preferred.
The Balmer series in the hydrogen spectrum corresponds to the transition from 1n = 2 to 2n = 3,4,.......... This series lies in the visible region. Calculate the wave number of line associated with the transition in Balmer series when the electron moves to n = 4 orbit. (RH = 109677 cm–1).
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We have V = 109677 [1/ni 2 - 1/nf 2 ] cm-1
Given, ni = 2, nf=4
V =109677 [1/4 - 1/16] = 20564.44 cm-1
According to de Broglie, matter should exhibit dual behaviour, that is both particle and wave like properties. However, a cricket ball of mass 100 g does not move like a wave when it is thrown by a bowler at a speed of 100 km/h. Calculate the wavelength of the ball and explain why it does not show wave nature.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: As per de Broglie, every object in motion has a wave character i.e. every object/matter have dual nature both particle and wave nature. But the wavelengths associated with ordinary objects are so short (because of their large masses) that their wave properties cannot be detected.
Given, m (mass) = 100g, v=100km/hr
We have
λ = h/mv= 238.5 × 10-34 m
As the wavelength associated with the cricket ball is so small that it can’t be detected.
What is the experimental evidence in support of the idea that electronic energies in an atom are quantized?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The line spectrum associated with any element possesses lines corresponding to specific wavelengths and these lines are obtained as a result of electronic transitions between the energy levels. Hence, the electrons in these levels have fixed energy i.e., quantized values.
Out of electron and proton which one will have, a higher velocity to produce matter waves of the same wavelength? Explain it.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We have
λ = h/mv
Thus, the equation signifies that in order to have the same wavelength the electron should have higher velocity as the mass of the proton is higher than that of the electron
A hypothetical electromagnetic wave is shown in Fig. 2.2. Find out the wavelength of the radiation
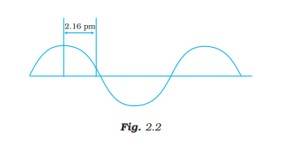
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The wavelength is defined as the distance between two consecutive crests or troughs of a wave, and it is denoted by l .
l = 4*2.16 pm = 8.64 pm
Chlorophyll present in green leaves of plants absorbs light at 4.620 × 1014 Hz. Calculate the wavelength of radiation in nanometers. Which part of the electromagnetic spectrum does it belong to?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We know that
λ= c/υ Given,
υ = 4.620 × 1014 Hz
Thus, λ= c/υ = (3.0 × 108 m/s)/ (4.620×1014 Hz) = 649.4nm
This frequency falls under visible range
What is the difference between the terms orbit and orbital?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:


Table-tennis ball has a mass 10 g and a speed of 90 m/s. If speed can be measured within an accuracy of 4% what will be the uncertainty in speed and position?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Given, m (mass) = 10g, v (speed) = 90 m/s and accuracy = 4%
Uncertainty in speed = 3.6 ms-1
Uncertainty in position = h/4 πmΔv = 6.626 × 10-34/4 × 3.14 × 10 × 3.6
=1.36 × 10-33m
The effect of uncertainty principle is significant only for motion of microscopic particles and is negligible for the macroscopic particles. Justify the statement with the help of a suitable example.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The uncertainty principle is significantly only for the microscopic particles and not for the macroscopic particles can be concluded by considering the following example. Let us consider a particle or an object of mass 1 milligram i.e. 10-6 kg Then its uncertainty can be calculated as,
? x ? v = 6.626 10-34 / 4x 3.14 106
= 10-28 m-2 s-1
Thus, the value obtained is negligible and insignificant for the uncertainty principle to be applied to this particle.
Hydrogen atom has only one electron, so mutual repulsion between electrons is absent. However, in multielectron atoms mutual repulsion between the electrons is significant. How does this affect the energy of an electron in the orbitals of the same principal quantum number in multielectron atoms?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The energy of electrons is determined by the value of n in the hydrogen atom and by n + l in the multielectron atom. Thus for a given principal quantum number the electrons of different orbitals would have different energy.
Class 11 Chemistry Structure of Atom NCERT Exemplar Long Answer Type Questions
The LA-type questions require well-structured and detailed answers. They help the students develop reasoning skills and deepen their in-depth understanding. Long Answer Type Questions challenge students to explain complex concepts like Schrödinger's wave equation, Heisenberg’s principle, de Broglie’s hypothesis, and Bohr’s theory.
LA Questions
| What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations. |
| ANS-When the beam of light gets exposed to the metal surface then the electrons get emitted from the metal. This effect is said to be photoelectric effect and the emitted electrons are said to be photoelectrons. The result of photoelectric experiment are as follows: - (i) There is no time gap between the striking of a light beam and the ejection of electrons from the metal surface i.e., electrons emit as soon as a beam of light strikes the metal surface. (ii) The number of electrons ejected is directly proportional to the intensity of light. (iii) For every metal there is minimum emitting frequency which is said to be threshold frequency, ν o .The kinetic energies of these electrons is directly proportional to the frequency of the light used. As per the quantum theory of electromagnetic radiation when a photon with energy greater than the threshold energy of the metal strikes the metal then the electrons get emitted from the metal without any delay and its kinetic energy depends upon the frequency of the light. As per the quantum theory of electromagnetic radiation when a photon with energy greater than the threshold energy of the metal strikes the metal then the electrons get emitted from the metal without any delay and its kinetic energy depends upon the frequency of the light |
| Threshold frequency, ν0 is the minimum frequency which a photon must possess to eject an electron from a metal. It is different for different metals. When a photon of frequency 1.0×1015 s–1 was allowed to hit a metal surface, an electron having 1.988 × 10–19 J of kinetic energy was emitted. Calculate the threshold frequency of this metal. Show that an electron will not be emitted if a photon with a wavelength equal to 600 nm hits the metal surface. |
| ANS- For the emission of electrons from metal the frequency of the striking light should be higher than that of its threshold frequency. We have h ν = hν 0 + K.E ν 0 = ν - K.E/h ……..(1) Given ν = 1015 s -1, K.E = 1.988 X 10-19J Thus (1) gives ν 0 = 7 x 1014 s -1 Given λ = 600 nm ν = c/ λ = 5 x 1014 s -1 As ν 0 > ν , thus the electrons do not emit. |
Commonly asked questions
Why was a change in the Bohr Model of atom required? Due to which important development (s), concept of movement of an electron in an orbit was replaced by, the concept of probability of finding electron in an orbital? What is the name given to the changed model of atom?
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The drawbacks of Bohr’s model were
(i) It was unable to explain the spectra for multi-electron systems
(ii) It could not explain the molecule formation through chemical bonds. The two important developments that contributed significantly towards the change of concept of movement of an electron in an orbit was replaced by, the concept of probability of finding an electron in an orbital were
(i) Dual nature of matter
(ii) Uncertainty Principle. Quantum mechanical model of the atom is the name of the new model.
When an electric discharge is passed through hydrogen gas, the hydrogen molecules dissociate to produce excited hydrogen atoms. These excited atoms emit electromagnetic radiation of discrete frequencies which can be given by the general formula
v= 109677 [1/ni 2 - 1/nf 2 ]
What points of Bohr’s model of an atom can be used to arrive at this formula? Based on these points derive the above formula giving description of each step and each term.
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: The points of the Bohr's model that can be consider are as follows: -
(i) Electrons revolve around the nucleus in a fixed orbit with fixed energy
(ii) The energy is absorbed or released when the electron moves from one energy level to another. The energy for the nth stationary state is given by
En = -2 p 2me4 /n2h2
Where
m = mass of the electron
e = charge of the electron
h= Planck's constant If an electron jumps from ni to nf then we have
D E= Ef - Ei = 2p2me4 /h2 [ (1/ni 2 ) - (1/nf2 )]
v = DE /hc = 109677 [ (1/ni 2 ) - (1/nf2 )]
Calculate the energy and frequency of the radiation emitted when an electron jumps from n = 3 to n = 2 in a hydrogen atom.
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: We have
v= 109677 [1/ni 2 - 1/nf 2 ]
Given, ni = 3 and nf =2
D E= hcv = 109677 [1/ni 2 - 1/nf 2 ]
DE = - 3.052 × 10-19J
n =DE /h= 4.606 × 1016Hz
Structure of Atom Class 11 Chemistry Matching Type Questions
To perform well in this section, the students need to have the ability to connect concepts like atomic models with their features, scientists with their discoveries, and quantum numbers with orbitals.
Matching Type Questions
Match the following species with their corresponding ground state electronic configuration.


This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Cu (c) 1s2 2s2 2p6 3s2 3p6 3d10 4s1
(ii) Cu2+ (d) 1s2 2s2 2p6 3s2 3p6 3d9
(iii) Zn2+ (a)1s2 2s2 2p6 3s2 3p6 3d10
(iv) Cr3+ (e) 1s2 2s2 2p6 3s2 3p6 3d3
Match the quantum numbers with the information provided by these.
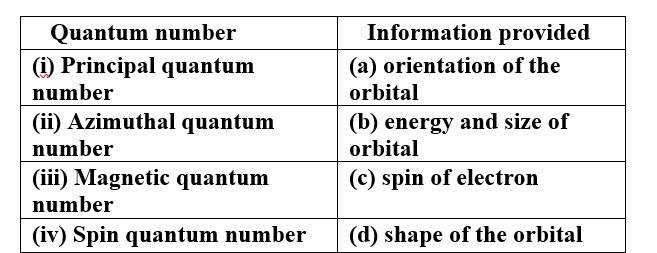
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Principal quantum number (b) energy and size of orbital
(ii) Azimuthal quantum number (d) shape of the orbital
(iii) Magnetic quantum number (a) orientation of the orbital
(iv) Spin quantum number (c) spin of electron
Commonly asked questions
Match the following rules with their statements :
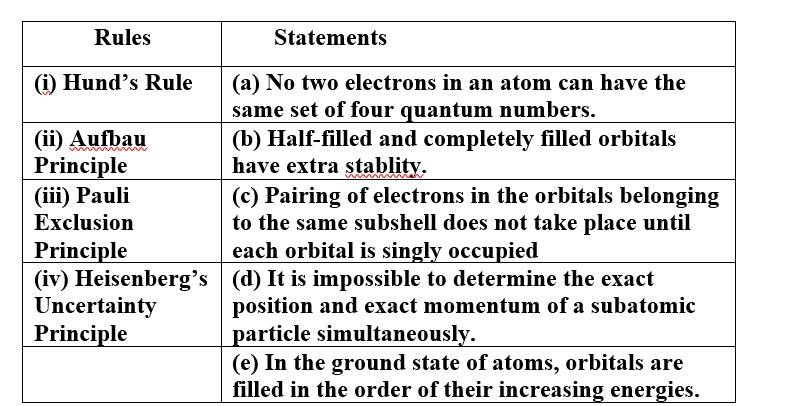
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Hund's Rule (c) Pairing of electrons in the orbitals belonging to the same subshell does not take place until each
orbital is singly occupied.
(ii) Aufbau Principle (e) In the ground state of atoms, orbitals are filled in the order of their increasing energies.
(iii) Pauli Exclusion (a) No two electrons in an atom can have the same set of four quantum numbers.
Principle
(iv) Heisenberg's (d) It is impossible to determine the exact position and exact momentum of a subatomic particle
Uncertainty simultaneously
Principle
Match the following
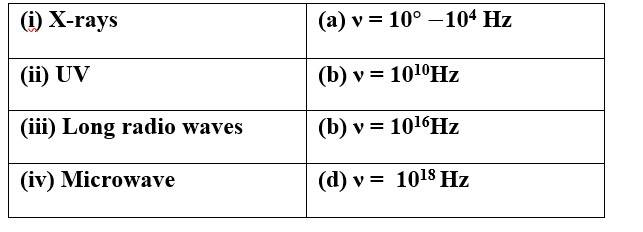
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) X-rays (d) ν = 1018Hz
(ii) UV (c) ν = 1016Hz
(iii) Long radio waves (a) ν = 10 -104 Hz
(iv)Microwave (b) ν = 1010 Hz
Match the following
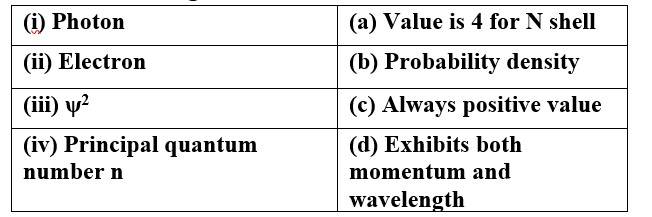
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Photon (d) Exhibits both momentum and wavelength
(ii) Electron (d) Exhibits both momentum and wavelength
(iii) ψ 2 (b) Probability density
(c) Always positive value
(iv) Principal quantum (a) Value is 4 for N shell
number n (c) Always positive value
Match species given in Column I with the electronic configuration given in Column II.
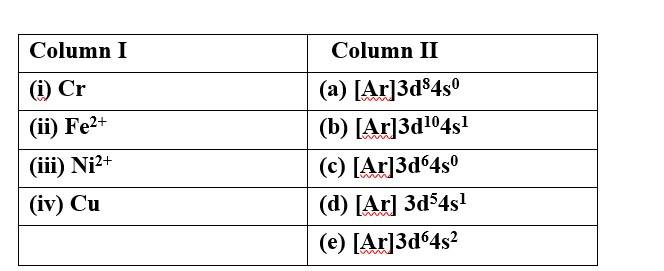
This is a Matching Type Questions as classified in NCERT Exemplar
Ans:
(i) Cr (d) [Ar] 3d54s2
(ii) Fe2+ (c) [Ar]3d64s0
(iii)Ni2+ (a) [Ar]3d84s0
(iv)Cu (b) [Ar]3d104s1
NCERT Exemplar Structure of Atom Class 11 Assertion and Reason Type
The Class 11 Assertion and Reason Type Questions of the Class 11 Structure of Atom evaluate the conceptual clarity and logical reasoning of the students. To answer these questions, they need to analyze the given statements and justify their correctness. This question helps in mastering the cause-effect relationships in atomic theory.
Assertion and Reason Type
Reason (R) : The chemical properties of an atom are controlled by the number of electrons in the atom.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A
(iii) A is true but R is false.
(iv) Both A and R are false.
Ans: option (i)
As the chemical properties depend upon the number of electrons present in the atom thus the isotopes resemble the same chemical properties as they have the same number of electrons i.e. atomic number.
Q: Assertion (A) : Black body is an ideal body that emits and absorbs radiations of all frequencies.
Reason (R) : The frequency of radiation emitted by a body goes from a lower frequency to higher frequency with an increase in temperature.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the explanation of A.
(iii) A is true and R is false.
(iv) Both A and R are false.
Ans: option (ii)
The black body is the ideal body that absorbs and emits radiation of all frequencies. The radiation that it emits is said to be black body radiation. With an increase of temperature, the frequency of the black body radiation increases.
Commonly asked questions
Assertion (A) : It is impossible to determine the exact position and exact momentum of an electron simultaneously.
Reason (R) : The path of an electron in an atom is clearly defined.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true and R is not the correct explanation of A.
(iii) A is true and R is false.
(iv) Both A and R are false.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: option (iii)
According to Heisenberg's uncertainty principle it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron. Thus, it implies that determining the trajectory of
an electron is impossible as it requires exact position and velocity which is not possible as per the uncertainty principle.
NCERT Exemplar Class 11 Chemistry Structure of Atom Objective Type Questions
The objective-type questions of the Structure of Atom Class 11 include multiple-choice questions that test the accuracy, recall of concepts, and conceptual application. They are especially useful for the competitive exam preparation. Students can do quick assessments by practicing the MCQs.
MCQs Below
(i) Most of the space in the atom is empty.
(ii) The radius of the atom is about 10–10 m while that of nucleus is 10–15 m.
(iii) Electrons move in a circular path of fixed energy called orbits.
(iv) Electrons and the nucleus are held together by electrostatic forces of attraction
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii)
Electrons move in a circular path of fixed energy called orbits .
As per Rutherford’s α-particle scattering experiment the nucleus is surrounded by electrons that move around the nucleus with a very high-speed in circular paths called orbits. It does not mention the energy or stability of the electrons revolving around the nucleus.
Which of the following options does not represent ground state electronic configuration of an atom?
(i) 1s2 2s2 2p6 3s2 3p6 3d8 4s2
(ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2
(iii) 1s2 2s2 2p6 3s2 3p6 3d10 4s1
(iv) 1s2 2s2 2p6 3s2 3p6 3d5 4s1
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2
As per the Hund's rule the half-filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half-filled 4s is preferred.
Commonly asked questions
Chlorine exists in two isotopic forms, Cl-37 and Cl-35 but its atomic mass is 35.5. This indicates the ratio of Cl-37 and Cl-35 is approximately
(i) 1:2
(ii) 1:1
(iii) 1:3
(iv) 3:1
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii) 1:3
The chlorine is a mixture of two isotopes with atomic masses 37U and 35U and its atomic mass is 35.5U. The atomic mass depicts that % composition of Cl-35 is much higher than that of Cl-37 and they are present in the ratio 1:3
The probability density plots of 1s and 2s orbitals are given in Fig. 2.1:
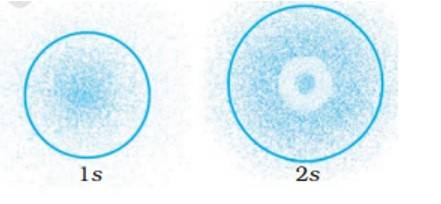
The density of dots in a region represents the probability density of finding electrons in the region.
On the basis of above diagram which of the following statements is incorrect?
(i) 1s and 2s orbitals are spherical in shape.
(ii) The probability of finding the electron is maximum near the nucleus.
(iii) The probability of finding the electron at a given distance is equal in all directions.
(iv) The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases.
This is a Multiple Choice Questions as classified in NCERT Exemplar
ANS – Option (iv)
The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases. Electrons at the 1s orbital decreases as we move far from the nucleus, however in case of 2s the probability decreases initially then it increases with the distance and thereafter at a certain point it starts decreasing with the distance.
Which of the following statement is not correct about the characteristics of cathode rays?
(i) They start from the cathode and move towards the anode.
(ii) They travel in straight line in the absence of an external electrical or magnetic field.
(iii) Characteristics of cathode rays do not depend upon the material of electrodes in cathode ray tube.
(iv) Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube. As per the result obtained from the cathode ray discharge tube experiment the characteristics of cathode rays (electrons) does not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube. Which concludes that electrons are the basic constituent of all the atoms
Which of the following statements about the electron is incorrect?
(i) It is a negatively charged particle.
(ii) The mass of electron is equal to the mass of neutron.
(iii) It is a basic constituent of all atoms.
(iv) It is a constituent of cathode rays.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
The mass of an electron is equal to the mass of a neutron.
The neutron is heavier than the electron as
Mass of the neutron = 1.67 x 10-27 kg
Mass of the electron = 9.11 x 10-31kg
Which of the following properties of atom could be explained correctly by Thomson Model of atom?
(i) Overall neutrality of atom.
(ii) Spectra of hydrogen atom.
(iii) Position of electrons, protons and neutrons in atom.
(iv) Stability of atom.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
Overall neutrality of atom.
According to the J.J Thomson model the positive charge is uniformly distributed and the electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement just as watermelon of positive charge with plums or seeds (electrons) embedded into it. Thus this model is able to explain the overall neutrality of the atom
Two atoms are said to be isobars if.
(i) They have same atomic number but different mass number.
(ii) They have same number of electrons but different number of neutrons.
(iii) They have same number of neutrons but different number of electrons.
(iv) Sum of the number of protons and neutrons is same but the number of protons is different.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
sum of the number of protons and neutrons is same but the number of protons is different
Isobars are the atoms which have same mass number (sum of number of neutrons and protons) but different atomic number (i.e. different proton number)
For example, 146C and 147N.
The number of radial nodes for 3p orbital is __________.
(i) 3
(ii) 4
(iii) 2
(iv) 1
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iv)
The number of radial nodes is given by n-l-1
where n is principal quantum number, l is azimuthal quantum number
For 3p orbital, n=3 and l=1
Thus, the number of radial nodes
= n-l-1
= 3-1-1
= 1
Number of angular nodes for 4d orbital is __________.
(i) 4
(ii) 3
(iii) 2
(iv) 1
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) 2
The number of angular nodes is given by n-l
where n is principal quantum number, l is azimuthal quantum number
For 4d orbital, n=4 and l=2
Thus, the number of angular nodes
= n-l
= 4-2
= 2
Which of the following is responsible to rule out the existence of definite paths or trajectories of electrons?
(i) Pauli’s exclusion principle.
(ii) Heisenberg’s uncertainty principle.
(iii) Hund’s rule of maximum multiplicity.
(iv) Aufbau principle
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (ii) Heisenberg's uncertainty principle.
According to Heisenberg's uncertainty principle it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron. Thus it implies that determining the trajectory of an electron is impossible as it requires exact position and velocity which is not possible as per the uncertainty principle.
Total number of orbitals associated with third shell will be __________.
(i) 2
(ii) 4
(iii) 9
(iv) 3
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) 9
The total number of orbitals is given by n2, where n is the principal quantum number or the principal shell.
Thus for the third shell number of orbitals is 32 i.e. 9
Orbital angular momentum depends on __________.
(i) l
(ii) n and l
(iii) n and m
(iv) m and s
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
“l” which is known as Azimuthal quantum number or orbital angular momentum or subsidiary quantum number depicts the three dimensional shape of the orbital.
The pair of ions having same electronic configuration is __________.
(i) Cr3+, Fe3+
(ii) Fe3+, Mn2+
(iii) Fe3+, Co3+
(iv) Sc3+, Cr3+
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii)
Fe3+, Mn2+ Fe3+ electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d5 4s2 Mn2+electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d5 4s
For the electrons of oxygen atom, which of the following statements is correct?
(i) Zeff for an electron in a 2s orbital is the same as Zeff for an electron in a 2p orbital.
(ii) An electron in the 2s orbital has the same energy as an electron in the 2p orbital.
(iii) Zeff for an electron in 1s orbital is the same as Zeff for an electron in a 2s orbital.
(iv) The two electrons present in the 2s orbital have spin quantum numbers ms but of opposite sign.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iv) The two electrons present in the 2s orbital have spin quantum numbers MS but of opposite sign.
According to Pauli’s Exclusion Principle no two electrons should possess the same set of four quantum numbers. Thus the two electrons present in the 2s orbital have spin quantum numbers MS but of opposite sign.
If travelling at same speeds, which of the following matter waves have the shortest wavelength?
(i) Electron
(ii) Alpha particle (He2+)
(iii) Neutron
(iv) Proton
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii) Alpha particle (He2+)
As the wavelength is inversely proportional to the mass of the particles thus the alpha particles would possess the shortest wavelength among the above given option
Identify the pairs which are not of isotopes?
(i) 12 6 X , 13 6Y
(ii) 35 17X, 37 17 Y
(iii) 146 X, 147Y
(iv) 84 X, 85Y
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) & (iv)
The isotopes are defined as atoms with identical atomic numbers but different mass numbers are known as isotopes.
Out of the following pairs of electrons, identify the pairs of electrons present in degenerate orbitals :
1. (a) n = 3, l = 2, ml = –2, ms=
(b) n = 3, l = 2, ml = –1, ms=
2. (a) n = 3, l = 1, ml = 1, ms = +
(b) n = 3, l = 2, ml = 1, ms = +
3. (a) n = 4, l = 1, ml = 1, ms = +
(b) n= 3, l = 2, ml = 1, ms = +
4. (a) n = 3, l = 2, ml = +2, ms = -
(b) n = 3, l = 2, ml = +2, ms = +
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i) & (iv)
In a multielectron atomic system the energy of an electron depends not only on its principal quantum number (shell), but also on its azimuthal quantum number (subshell). Electrons having the same shells and same subshells have the same energy and they are known as degenerate orbitals.
Which of the following sets of quantum numbers are correct?
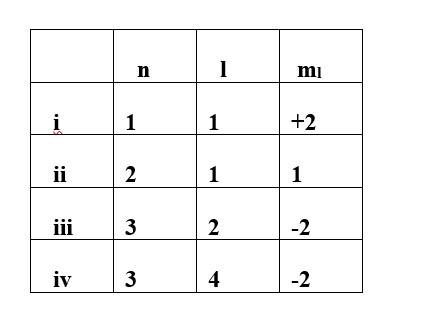
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (ii) & (iii)
For the given value of n (principal quantum number) the value of l (Azimuthal quantum number) varies from 0 to n-1. However for the given value of l the ml (magnetic quantum number) varies from -1 to +1.
In which of the following pairs, the ions are iso-electronic?
(i) Na+, Mg2+
(ii) Al3+, O–
(iii) Na+ , O2–
(iv) N3–, Cl–
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i) & (iii)
Isoelectronic species are those species that possess the same number of electrons. Here both Na+, Mg2+ possess 10 electrons and both Na+, O2– possess 10 electrons.
Which of the following statements concerning the quantum numbers are correct?
(i) Angular quantum number determines the three dimensional shape of the orbital.
(ii) The principal quantum number determines the orientation and energy of the orbital.
(iii) Magnetic quantum number determines the size of the orbital.
(iv) Spin quantum number of an electron determines the orientation of the spin of electron relative to the chosen axis.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (i)& (iv)
- Principal quantum number (n): It represents the size and energy of orbitals.
- Azimuthal quantum number (l): It represents the subshell and shape of orbitals.
- Magnetic quantum number (ml): It represents the orientation of the orbitals.
- Spin quantum number (ms): It represents the direction of spin of electrons in the orbitals.
FAQs Related to Chemistry Chapter Two NCERT Exemplar
Following are the frequently asked questions of this chapter:
Commonly asked questions
In atomic structure, what are the quantum numbers and why they are important?
Quantum numbers describe the unique position and energy of an electron in an atom. These are a set of four numbers and are important for predicting chemical behavior and understanding the distribution of electrons in orbitals.
In multi-electron atoms, why is Bohr’s model not applicable?
The hydrogen atom can be accurately explained by Bohr's model. However, it does not account for shielding effects, electron-electron interactions, and the wave nature of electrons for multi-electron atoms.
In atomic structure, what is the significance of the de Broglie equation?
According to the de Broglie equation, the particles like electrons have wave-like properties. The quantum mechanical model foundation is laid by this concept and it also explains the stability of electron orbits using wave behavior.
Common Mistakes and Tips for NCERT Chemistry Exemplar Chapter Two
JEE Mains 2022
Commonly asked questions
The normality of H2SO4 in the solution obtained on mixing 100 mL of 0.1 M H2SO4 with 50 mL of 0.1 M NaOH is__________× 10-1N. (Nearest Integer)
No. of equivalents of H2SO4 = 100 × 0.1 × 2 = 20
No. of equivalents of NaOH = 50 × 0.1 = 5
No. equivalents of H2SO4 left = 20 – 5 = 15
150 × x = 15
For a real at 25°C temperature and high pressure (99 bar) the value of compressibility factor is 2, so the value of Vander Waal’s constant ‘b’ should be__________× 10-2 L mol-1. (Nearest Integer)
(Given R = 0.083 L bar K-1 mol-1)
For real gas under high pressure,
= 0.25 × 10-2 L mol-1
A gas (Molar mass = 280 g mol-1) was burnt in excess O2 in a constant volume calorimeter and during combustion the temperature of calorimeter increased from 298.0 K to 298.45K. If the heat capacity of calorimeter is 2.5 kJ K-1 and enthalpy of combustion of gas is 9 kJ mol-1 the amount of gas burnt is _____________g. (Nearest Integer)
Let x gm is burnt
Moles = x/280
Heat released by x/280 mol = 2.5 × 0.45 kJ
Heat released by 1 mol =
x = 35 gm
When a certain amount of solid A is dissolved in 100g of water at 25°C to make a dilute solution, the vapour pressure of the solution is reduced to one-half of that of pure water. The vapour pressure of pure water is 23.76 mmHg. The number of moles of solute A added is_________.
(Nearest Integer)
[A] -> [B]
Reactant Product
If formation of compound [B] follows the first order of kinetics and after 70 minutes the concentration of [A] was found to be half of its initial concentration. Then the rate constant of the reaction is x × 10-6 s-1. The value of x is___________. (Nearest Integer)
= 165 × 10-6 s-1
Among the following ores Bauxite, Siderite, Cuprite, Calamine, Haematite, Kaolinite, Malachite, Magnetite, Sphalerite, Limonite, Cryolite, the number of principal ores if iron is ___________.
Bauxite = AlOx (OH)3-2x (where O < x < 1)
Siderite = FeCO3
Cuprite = Cu2O
Calamine = ZnCO3
Haemetite = Fe2O3
Kaolinite = Al2 (OH)4Si2O5
Malachite = CuCO3 Cu (OH)2
Magneite = Fe3O4
Sphalerite = ZnS
Limonite = Fe2O3.3H2O
The oxidation state of manganese in the product obtained in a reaction of potassium permanganate and hydrogen peroxide in basic medium is___________.
The number of molecule(s) or ion(s) from the following having non-polar structure is _________.
SO3 -> Sp2 Planar
BF3 -> Sp2 Planar
-> Sp2 Planar
SF4 -> Sp3d non-planar
H2O2 -> Sp3 Non-planar
PCl3 -> Sp3 Non –planar
[Al (OH)4]- -> Sp3 Non-planar
XeF4 -> Sp3d2 planar
XeO3 -> Sp3 Non-planar
-> Sp3 Non-planar
The spin only magnetic moment of the complex present in Fehling’s reagent is __________B.M.
(Nearest Integer)
Fehling's solution is a complex of
No. of unpaired e- = 1
MM =
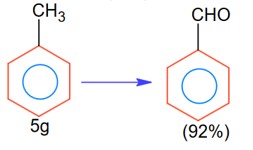
In the below reaction, 5g of toluene is converted into benzaldehyde with 92% yield. The amount of benzaldehyde produced is____________× 10-2 g. (Nearest integer)
No. of moles =
Moles of benzaldehyde =
Mass of benzaldehyde = 5 × 10-2 ×106 = 5.3 gm
= 530 × 10-2 gm
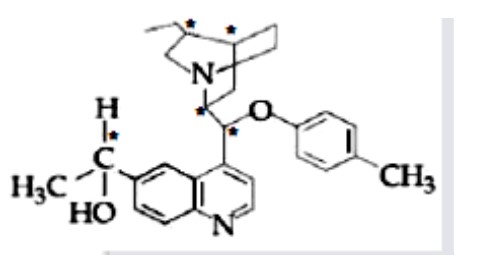
The number of chiral carbons present in the molecule given below is
Kindly consider the following Image
The Gibbs energy change (in J) for the given reaction at [Cu²?] = [Sn²?] = 1M and 298 K is Cu(s) + Sn²?(aq) → Cu²?(aq) + Sn(s); (E?Sn²?/Sn = -0.16V, E?Cu²?/Cu = 0.34 V)
Take F = 96500 Cmol?¹
Sol. E? cell = E? (Sn²? |Sn) - E? (Cu²? |Cu)
= -0.16 - 0.34 = -0.50V
ΔG? = -nFE? cell
= -2 × 96500 × (-0.5) = 96500 J
= 96.5 kJ = 96500 J
The oxidation states of iron atoms in compounds (A), (B) and (C), respectively, are x, y and z. The sum of x,y and z is [numerical value].
(A) Na?[Fe(CN)?(NOS)], (B) Na?[FeO?], (C) [Fe?(CO)?]
The oxidation states of iron in these compounds will be
A = +2
B = +4
C = 0
The sum of oxidation states will be = 6.
The internal energy change (in J) when 90 g of water undergoes complete evaporation at 100°C is
(Given: ΔH_vap for water at 373K = 41 kJ/mol, R = 8.314 JK?¹mol?¹)
189000 to 190000
Sol. ΔH = ΔU + Δn? RT
41000 × 5 = ΔU + 5 × 8.314 × 373
205000 = ΔU + 15505.61
ΔU = 189494.39 J = 189494 J
The mass of gas adsorbed, x, per unit mass of adsorbate, m, was measured at various pressures, p. A graph between log(x/m) and log p gives a straight line with slope equal to 2 and the intercept equal to 0.4771. The value of x/m at a pressure of 4 atm is: [numerical value].
(Given log3 = 0.4771)
Sol. (x/m) = k (P)¹/?
log (x/m) = logk + 1/n logP
Slope = 1/n = 2 So n = 1/2
Intercept ⇒ logk = 0.477 So k = Antilog (0.477) = 3
So (x/m) = k (P)¹/? = 3² = 48
Chemistry NCERT Exemplar Solutions Class 11th Chapter Two Exam