Structure of Atoms
Get insights from 148 questions on Structure of Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (ii) 1s2 2s2 2p6 3s2 3p6 3d9 4s2
As per the Hund's rule the half-filled and fully filled orbital leads to the extra stability due to the symmetry thus fully filled 3d and half-filled 4s is preferred.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iii)
Electrons move in a circular path of fixed energy called orbits .
As per Rutherford's α-particle scattering experiment the nucleus is surrounded by electrons that move around the nucleus with a very high-speed in circular paths called orbits. It does not mention the energy or stability of the electrons revolving around the nucleus.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The energy of electrons is determined by the value of n in the hydrogen atom and by n + l in the multielectron atom. Thus for a given principal quantum number the electrons of different orbitals would have different energy.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The uncertainty principle is significantly only for the microscopic particles and not for the macroscopic particles can be concluded by considering the following example. Let us consider a particle or an object of mass 1 milligram i.e. 10-6 kg Then its uncertainty can be calculated as,
? x ? v = 6.626 10-34 / 4x 3.14 106
= 10-28 m-2 s-1
Thus, the value obtained is negligible and insignificant for the uncertainty principle to be applied to this particle.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: Given, m (mass) = 10g, v (speed) = 90 m/s and accuracy = 4%
Uncertainty in speed = 3.6 ms-1
Uncertainty in position = h/4 πmΔv = 6.626 * 10-34/4 * 3.14 * 10 * 3.6
=1.36 * 10-33m
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans:


New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We know that
λ= c/υ Given,
υ = 4.620 * 1014 Hz
Thus, λ= c/υ = (3.0 * 108 m/s)/ (4.620*1014 Hz) = 649.4nm
This frequency falls under visible range
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
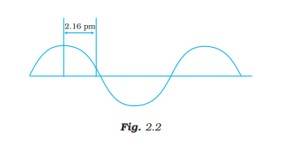
Ans: The wavelength is defined as the distance between two consecutive crests or troughs of a wave, and it is denoted by l .
l = 4*2.16 pm = 8.64 pm
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: We have
λ = h/mv
Thus, the equation signifies that in order to have the same wavelength the electron should have higher velocity as the mass of the proton is higher than that of the electron
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: The line spectrum associated with any element possesses lines corresponding to specific wavelengths and these lines are obtained as a result of electronic transitions between the energy levels. Hence, the electrons in these levels have fixed energy i.e., quantized values.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers