Structure of Atoms
Get insights from 148 questions on Structure of Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Structure of Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
“l” which is known as Azimuthal quantum number or orbital angular momentum or subsidiary quantum number depicts the three dimensional shape of the orbital.
New answer posted
7 months agoTotal number of orbitals associated with third shell will be __________.
(i) 2
(ii) 4
(iii) 9
(iv) 3
Contributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) 9
The total number of orbitals is given by n2, where n is the principal quantum number or the principal shell.
Thus for the third shell number of orbitals is 32 i.e. 9
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (ii) Heisenberg's uncertainty principle.
According to Heisenberg's uncertainty principle it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron. Thus it implies that determining the trajectory of an electron is impossible as it requires exact position and velocity which is not possible as per the uncertainty principle.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iii) 2
The number of angular nodes is given by n-l
where n is principal quantum number, l is azimuthal quantum number
For 4d orbital, n=4 and l=2
Thus, the number of angular nodes
= n-l
= 4-2
= 2
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (iv)
The number of radial nodes is given by n-l-1
where n is principal quantum number, l is azimuthal quantum number
For 3p orbital, n=3 and l=1
Thus, the number of radial nodes
= n-l-1
= 3-1-1
= 1
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
sum of the number of protons and neutrons is same but the number of protons is different
Isobars are the atoms which have same mass number (sum of number of neutrons and protons) but different atomic number (i.e. different proton number)
For example, 146C and 147N.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (i)
Overall neutrality of atom.
According to the J.J Thomson model the positive charge is uniformly distributed and the electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement just as watermelon of positive charge with plums or seeds (electrons) embedded into it. Thus this model is able to explain the overall neutrality of the atom
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (ii)
The mass of an electron is equal to the mass of a neutron.
The neutron is heavier than the electron as
Mass of the neutron = 1.67 x 10-27 kg
Mass of the electron = 9.11 x 10-31kg
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: Option (iv)
Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube. As per the result obtained from the cathode ray discharge tube experiment the characteristics of cathode rays (electrons) does not depend upon the material of electrodes and the nature of the gas present in the cathode ray tube. Which concludes that electrons are the basic constituent of all the atoms
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
ANS – Option (iv)
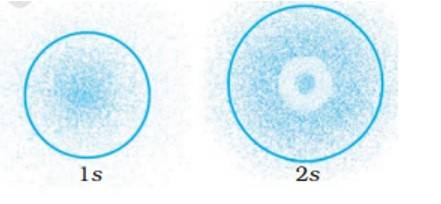
The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases. Electrons at the 1s orbital decreases as we move far from the nucleus, however in case of 2s the probability decreases initially then it increases with the distance and thereafter at a certain point it starts decreasing with the distance.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers